Discussion
Our findings suggest that the D1.1 outbreak in Nevada was caused by a single introduction of the D1.1 genotype viruses into cattle. However, the four available cattle D1.1 virus genomes appear to come from a single herd, and we do not yet know the extent of this outbreak or the amount of viral diversity currently among the cattle.
Additional sampling of D1.1 viruses from cattle in other infected herds in Churchill County, as well as from birds and other mammals infected with close relatives, may fill in some of the long period of unsampled genetic diversity represented by the long “stem” branch of the D1.1 cattle clade (driving the cattle clade tMRCA earlier and the tMRCA of that clade and its closest sampled relatives later). Though on a different time scale, a similarly long branch—±indicative of significant gaps in surveillance—was observed between the human viral genome sequences and the common ancestor they shared with the most closely related swine viruses available in data repositories at the outset of the 2009 pandemic H1N1 (15). A communication from APHIS (4) notes that, in Churchill County Nevada, “the affected dairy producers reported large wild bird die-offs near the dairies”. If these birds were infected with the same lineage of D1.1 as the cattle, then genomes from D1.1 viruses sampled from these birds could help further narrow the plausible date of introduction of D1.1 into cattle. To understand the frequency and risks of mammalian emergence of 2.3.4.4b viruses it remains critically important that dead or morbid wild birds observed in the vicinity of infected cattle herds, before and after H5 detection in cattle, are tested for HPAIV.
The presence of PB2 D701N (nucleotide substitution G2101A; Figure 8) in all four cattle D1.1 genomes (28) suggests a period of evolution within cattle on the stem branch of the four cattle D1.1 viruses (i.e., the purple branch in Figures 2, 3 and 6). This is consistent with the molecular clock analysis that also suggests a period of evolution within cattle, along the stem branch for that clade in our phylogenetic analyses (Figure 7C). The D701N mutation frequently emerges in H5N1 2.3.4.4b viruses following spillover into mammals (e.g., in fox, lynx, and seal) but, to our knowledge, has not been observed in wild birds infected with 2.3.4.4b, except for a few rare cases in South America where spillback from marine mammals to shorebirds is proposed.
These findings demonstrate the utility of the National Milk Testing Strategystrong text1strong text (NMTS) for identifying novel introductions of H5N1 into dairy cattle. The NMTS was rolled out by APHIS/USDA in December 2024 to provide a more systematic means of testing milk nationally to monitor the outbreak’s spread and identify infected herds. As of February 16, 2025, 42 US states are enrolled in NMTS and conducting active surveillance (1). This detection of D1.1 in milk silo samples from Nevada demonstrates the importance of a systematic, ongoing national milk surveillance approach for early detection, as this second spillover of H5N1 into cattle might not have been identified otherwise.
On January 24th, 2025, the Nevada State Department of Agriculture announced the first quarantines of Churchill County dairy premises (29). (The December 6th, 2024 announcement of quarantines of dairy premises in Nye County, some 250 miles from Churchill County (30), involved a separate (B3.13) outbreak unrelated to the current D1.1 outbreak.) Thus, the time between initial silo sample collection and the first quarantine control measures was 18 days. Our findings are consistent with the quarantines occurring long after spillover, perhaps a month or more.
Encouragingly, the Nevada State Department of Agriculture has communicated that “there have been no movements of dairy cattle within dairies in Nevada, nor any new animals introduced to any dairies within the affected area reported within 30 days prior to the first silo detection. The only movement of cattle out of the affected area are cull cows that have moved directly to slaughter.”
The National Silo Monitoring Program provides a powerful, accessible, and convenient means to detect influenza virus in dairy cattle herds across the country. But it is critical that we close the gaps between outbreak initiation and first detection, and first detection and control measures like quarantines being put into place. The ability to perform the genomic epidemiological analyses, provided by the release of sequencing data from these d1.1-infected cattle by APHIS, and reveals that it may have been more than a month from spillover to quarantines being put into place on the first two herds confirmed to have HPAI in the Northern Nevada outbreak. And as of February 21, 2025 there are now 7 herds confirmed to be infected in this outbreak (31). This may therefore be a case of closing the barn door after the cow has bolted. For both the National Silo Monitoring Program and the on-farm bulk milk testing programs within the National Milk testing Strategy, we propose the following ideas to shorten the gap between new HPAI introductions to cattle herds and control measure being initiated:
● Immediately place quarantines on all herds that may have contributed milk to a silo that tests positive, then relax quarantines if follow-up testing of samples from individual herds are influenza virus-negative.
● To avoid unnecessary disruptions that the above point would cause, archive, on a rolling basis, small volumes (e.g. 1 L) of milk from each shipment from each farm that is delivered to each silos at processing facilities so that samples from individual premises/herds are available immediately for follow-up testing should a silo test positive.
● For silos, whose large volume might dilute virus present in the milk of a single herd or even a single cow, concentrate virus from a large volume of milk for each test to reduce false-negative influenza A virus PCR results caused by dilution by uninfected milk of infected milk, which will initially rare.
● Develop and employ inexpensive, field-deployable, sensitive rapid tests to avoid delays caused by shipping samples off site and waiting for lab test results, and to allow more frequent testing.
● Where possible, test milk from individual herds to prevent delays caused by having to collect new samples from multiple farms that may have contributed to a positive silo test.
A major driver of the extensive spread of the B3.13 virus through the cattle population was the inability to ascertain the full scope and scale of the outbreak and institute quarantine and animal movement restrictions early on, before the virus transmitted onward to additional herds. The National Milk Testing Strategy and continued testing (and surveillance of wild and domestic birds) are therefore essential to controlling the current cattle epizootic. If the D1.1 lineage has spilled over into a part of the US dairy cattle network previously untouched by, or at least less affected by, the spread of B3.13, then the D1.1 lineage has the potential to result in a large outbreak, like B3.13. The D1.1 genotype is now highly prevalent not only in the US, but also in Canada (e.g., British Columbia), so it is reasonable to anticipate that additional introductions of this genotype to cattle might occur, or might already have occurred, not only in the US but also Canada. Our results highlight the critical importance of surveillance of wild animal monitoring viral spillover into high density populations of agricultural animals more generally which can serve to rapidly amplify and spread pathogens heightening risk of spillover into humans.
Acknowledgements
We thank APHIS/USDA for generating and sharing sequence reads data from the four cattle infected with D1.1. And we thank all who contributed to the milk testing efforts that led to the discovery of this outbreak in cattle and, ultimately, the generation and release of these sequencing data: the dairy producers and farm workers of Churchill County, USDA, APHIS, the National Veterinary Services Laboratories, the National Milk Testing Strategy, the National Silo Monitoring Program, the Washington Animal Disease Diagnostic Laboratory, the Nevada State Department of Agriculture and other State and local officials. We additionally thank the Nevada State Department of Agriculture for information communicated to M.W. about recent cattle movements into or out of Churchill County. We thank the British Columbia Centre for Disease Control for sharing multiple avian influenza virus complete genomes used in our analyses. And we appreciate comments provided by Florence Débarre.
GISAID: We are very grateful to all the laboratories and organizations who have generated and made sequences available that have allowed these inferences in a timely fashion. We gratefully acknowledge all data contributors, i.e., the Authors and their Originating laboratories responsible for obtaining the specimens, and their Submitting laboratories for generating the genetic sequence and metadata and sharing via the GISAID Initiative, on which this research is based. Our GISAID EPI_SET ID is EPI_SET_250220xk and associated doi: 10.55876/gis8.250220xk. Those without GISAID Access Credentials may retrieve information about all contributors of data on which our analysis is based by either clicking on the DOI or pasting the EPI_SET ID in the “Data Acknowledgement Locator” on the GISAID homepage. Accession numbers and metadata of all sequences included in our analyses are provided in Table 5 available via Final_all-D1.1-FEB20-sequence-metadata-n245.pdf - Google Drive.
Addendum
As we were finalizing this report, we learned of two relevant discoveries. First, D1.1 clade 2.3.4.4b H5N1 was detected in on-farm bulk milk samples from one dairy cattle herd in Arizona (32). This was reportedly a distinct introduction from birds to cattle and not directly related to the Nevada cattle outbreak (32). That herd has been quarantined, and it will be critical to see whether the Arizona outbreak has remained isolated to a single herd. The potentially quicker discovery of the Arizona outbreak via on-farm milk sampling, rather than dairy processing plant silo sample collection with subsequent follow-up sampling and additional testing of individual herds in Nevada, could contribute to more effective control.
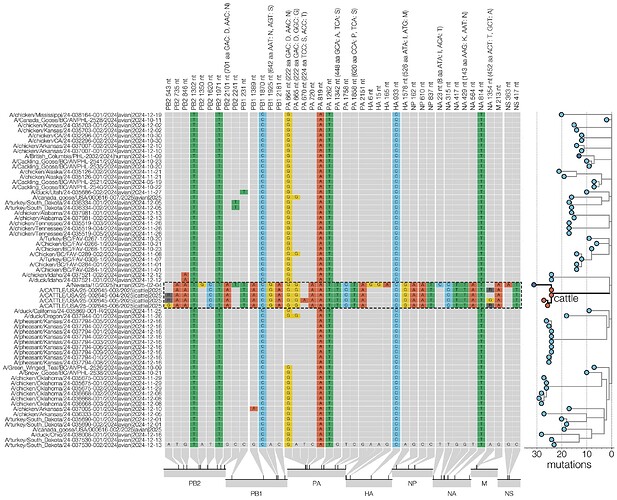
Addendum Figure 1 - Condensed alignment of genome positions variable in cattle when including the human viral genome sequence from the recent case in Nevada. D1.1 sequences (outlined with a dashed line) compared to alignment consensus (left). For clarity only a subtree containing the cattle and human D1.1 sequences is shown here (right, branch lengths scaled to number of substitutions). Relative positions of each site variable in cattle D1.1 sequences are shown at the bottom.
Second, the complete viral genome sequence from a human case associated with the Nevada D1.1 cattle outbreak was shared by the Nevada State Health Laboratory and the US Centers for Disease and Control and Prevention. This genome clusters with the Nevada D1.1 cattle virus genomes and possesses all the cattle clade-identifying SNPs, as well as several additional substitutions in HA (Addendum Figure 1).
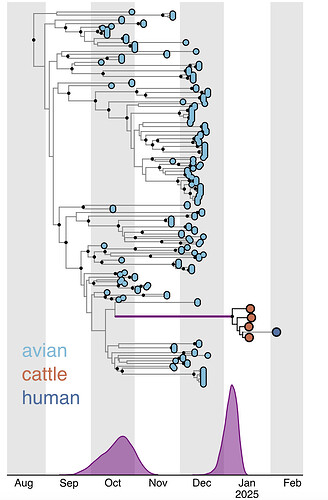
As this human virus clusters with the cattle clade, we have repeated the phylogenetic analysis using an exponential population size coalescent prior and a fixed local clock on the cattle clade, now with the additional human virus genome. We again modelled the assignment of the stem branch to either the avian rate or the cattle rate, using Bayesian search variable selection. Here, we assume that the branch leading to the human virus represents evolution in cattle (i.e., evolves at the same rate as in cattle), which is reasonable considering that no human-to-human transmission of cattle-origin viruses has to our knowledge been reported.
Addendum Figure 2 - Temporal phylogeny of 2.3.4.4b D1.1 genomes when including the human viral genome sequence from the recent case in Nevada. The phylogeny includes all avian virus genomes with complete sampling dates, the four genomes sampled from cattle, and the human virus genome from the recent case in Nevada. The time during which the D1.1 lineage spilled over into cattle is represented by the thicker purple branch. The start (left) node and end (right) node of this branch represent, respectively, the MRCA of the cattle viruses and the most closely related taxa, and the MRCA of the cattle viruses. The times of these MRCAs are indicated by purple KDE plots, and the median and 95% HPDare listed in Addendum Table 1.
We find that the human virus genome clusters with the cattle clade (P>0.999), and the tMRCA of the cattle clade has shifted slightly earlier (median=2025-01-05; 95% HPD: 25 December 2024 – 12 January 2025; Addendum Figure 2) compared to otherwise identical analyses that did not include the human viral genome sequence. The tMRCA of the cattle clade and the most closely related avian viruses was consistent with whether the human virus genome was included or not (Addendum Table 1, Table 3). Our results, therefore, define a consistent time frame during which the D1.1 lineage spilled over from birds into cattle, likely between mid-late October 2024 and late December 2024 or early January 2025. We again find low support for the entire branch leading to the MRCA of the cattle clade to have evolved at the cattle rate (P=0.239), and our estimate for when D1.1 spilled over into cattle is just slightly later, now at 6 December 2025.
References
| 1. | National Milk Testing Strategy [Internet]. Animal and Plant Health Inspection Service. [cited 2025 Feb 7];Available from: National Milk Testing Strategy | Animal and Plant Health Inspection Service |
|---|---|
| 2. | CDC. Genetic Sequences of Highly Pathogenic Avian Influenza A(H5N1) Viruses Identified in a Person in Louisiana [Internet]. Avian Influenza (Bird Flu). 2024 [cited 2025 Feb 9];Available from: Genetic Sequences of Highly Pathogenic Avian Influenza A(H5N1) Viruses Identified in a Person in Louisiana | Bird Flu | CDC |
| 3. | Dyer O. Bird flu: Canadian teenager is critically ill with new genotype. BMJ [Internet] 2024;387:q2529. Available from: http://dx.doi.org/10.1136/bmj.q2529 |
| 4. | [No title] [Internet]. [cited 2025 Feb 8];Available from: https://www.aphis.usda.gov/sites/default/files/dairy-cattle-hpai-tech-brief.pdf |
| 5. | New avian influenza genotype found in dairy cattle [Internet]. American Veterinary Medical Association. [cited 2025 Feb 7];Available from: https://www.avma.org/news/new-avian-influenza-genotype-found-dairy-cattle |
| 6. | [No title] [Internet]. [cited 2025 Feb 12];Available from: https://www.aphis.usda.gov/sites/default/files/dairy-cattle-hpai-tech-brief.pdf |
| 7. | [No title] [Internet]. [cited 2025 Feb 12];Available from: https://www.aphis.usda.gov/sites/default/files/dairy-cattle-hpai-tech-brief.pdf |
| 8. | Community Alerts - Week of February 10, 2025 [Internet]. Central Nevada Health District. 2024 [cited 2025 Feb 13];Available from: Community Alerts - Week of February 10, 2025 - Central Nevada Health District |
| 9. | OverviewOfTheGradeADairyProgram [Internet]. [cited 2025 Feb 10];Available from: https://agri.nv.gov/Food/Food_Safety/Safety/OverviewOfTheGradeADairyProgram/ |
| 10. | Grubaugh ND, Gangavarapu K, Quick J, et al. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol [Internet] 2019;20(1):8. Available from: An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar | Genome Biology | Full Text |
| 11. | Worobey M, Han G-Z, Rambaut A. A synchronized global sweep of the internal genes of modern avian influenza virus. Nature [Internet] 2014;508(7495):254–7. Available from: A synchronized global sweep of the internal genes of modern avian influenza virus | Nature |
| 12. | [No title] [Internet]. [cited 2025 Feb 12];Available from: https://www.aphis.usda.gov/sites/default/files/dairy-cattle-hpai-tech-brief.pdf |
| 13. | Preliminary report on genomic epidemiology of the 2024 H5N1 influenza A virus outbreak in U.S. cattle (Part 1 of 2) [Internet]. Virological. 2024 [cited 2025 Feb 13];Available from: Preliminary report on genomic epidemiology of the 2024 H5N1 influenza A virus outbreak in U.S. cattle (Part 1 of 2) |
| 14. | Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol [Internet] 2018;4(1):vey016. Available from: http://dx.doi.org/10.1093/ve/vey016 |
| 15. | Uhart MM, Vanstreels RET, Nelson MI, et al. Epidemiological data of an influenza A/H5N1 outbreak in elephant seals in Argentina indicates mammal-to-mammal transmission. Nature Communications [Internet] 2024 [cited 2025 Feb 10];15(1):1–14. Available from: Epidemiological data of an influenza A/H5N1 outbreak in elephant seals in Argentina indicates mammal-to-mammal transmission | Nature Communications |
| 16. | [No title] [Internet]. [cited 2025 Feb 10];Available from: https://www.aphis.usda.gov/sites/default/files/dairy-cattle-hpai-tech-brief.pdf |
| 17. | Gabriel G, Dauber B, Wolff T, Planz O, Klenk HD, Stech J. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proceedings of the National Academy of Sciences of the United States of America [Internet] 2005 [cited 2025 Feb 8];102(51). Available from: The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host - PubMed |
| 18. | Website [Internet]. Available from: Isolation and Characterization of Novel Reassortant Influenza A(H10N7) Virus in a Harbor Seal, British Columbia, Canada - PMC, Emergence of Fatal Avian Influenza in New England Harbor Seals - PMC, Detection of H3N8 influenza A virus with multiple mammalian-adaptive mutations in a rescued Grey seal (Halichoerus grypus) pup - PubMed |
| 19. | Dunham EJ, Dugan VG, Kaser EK, et al. Different Evolutionary Trajectories of European Avian-Like and Classical Swine H1N1 Influenza A Viruses. Journal of Virology [Internet] 2009 [cited 2025 Feb 8];83(11):5485. Available from: Different Evolutionary Trajectories of European Avian-Like and Classical Swine H1N1 Influenza A Viruses - PMC |
| 20. | Website [Internet]. Available from: https://academic.oup.com/ve/article/10/1/veae031/7645834 |
| 21. | Website [Internet]. Available from: https://journals.asm.org/doi/10.1128/jvi.00213-23 |
| 22. | Website [Internet]. Available from: Multiple polymerase gene mutations for human adaptation occurring in Asian H5N1 influenza virus clinical isolates | Scientific Reports |
| 23. | Gabriel G, Herwig A, Klenk HD. Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus. PLoS pathogens [Internet] 2008 [cited 2025 Feb 8];4(2). Available from: Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus - PubMed |
| 24. | Staller E, Carrique L, Swann OC, et al. Structures of H5N1 influenza polymerase with ANP32B reveal mechanisms of genome replication and host adaptation. Nature communications [Internet] 2024 [cited 2025 Feb 8];15(1). Available from: Structures of H5N1 influenza polymerase with ANP32B reveal mechanisms of genome replication and host adaptation - PubMed |
| 25. | Dadonaite B, Ahn JJ, Ort JT, et al. Deep mutational scanning of H5 hemagglutinin to inform influenza virus surveillance. PLoS Biol [Internet] 2024;22(11):e3002916. Available from: Deep mutational scanning of H5 hemagglutinin to inform influenza virus surveillance |
| 26. | Peacock TP, Moncla L, Dudas G, et al. The global H5N1 influenza panzootic in mammals. Nature [Internet] 2025;637(8045):304–13. Available from: The global H5N1 influenza panzootic in mammals | Nature |
| 27. | Xie R, Edwards KM, Wille M, et al. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature [Internet] 2023 [cited 2025 Feb 12];622(7984). Available from: The episodic resurgence of highly pathogenic avian influenza H5 virus - PubMed |
| 28. | [No title] [Internet]. [cited 2025 Feb 10];Available from: https://www.aphis.usda.gov/sites/default/files/dairy-cattle-hpai-tech-brief.pdf |
| 29. | Highly Pathogenic Avian Influenza detected in dairy cows in northern Nevada [Internet]. [cited 2025 Feb 9];Available from: Highly Pathogenic Avian Influenza detected in dairy cows in northern Nevada |
| 30. | First Highly Pathogenic Avian Influenza detection in dairy cows in Nevada [Internet]. [cited 2025 Feb 9];Available from: First Highly Pathogenic Avian Influenza detection in dairy cows in Nevada |
| 31. | HPAI Confirmed Cases in Livestock [Internet]. Animal and Plant Health Inspection Service. [cited 2025 Feb 21];Available from: HPAI Confirmed Cases in Livestock | Animal and Plant Health Inspection Service |
| 32. | Milk from Maricopa County dairy cows tested positive for bird flu [Internet]. ABC15 Arizona in Phoenix (KNXV). 2025 [cited 2025 Feb 17];Available from: https://www.abc15.com/news/local-news/milk-from-maricopa-county-dairy-cows-tested-positive-for-bird-flu |