The SARS-CoV-2 variant Delta displaced the variants Gamma and Gamma plus in Amazonas, Brazil
Felipe Gomes Naveca 1,2, Valdinete Nascimento 1, Victor Souza 1, André de Lima Corado 1, Fernanda Nascimento 1, Matilde Mejía 1, Maria Júlia Brandão 1, Arlesson Viana da Silva 1, Adele Schwartz Benzaken 3, George Silva 1,4, Luciana Gonçalves 1,5, Sérgio Luiz Bessa Luz 1, José Joaquín Carvajal Cortés 1, Juan Camilo Grisales Nieto 1, Kelly Natalia Romero Vesga 1, Cristiano Fernandes 5, Tatyana Amorim 5, Tirza Mattos 6, Ligia Abdalla 7, João Hugo Santos 8, Gabriel Luz Wallau 9, Edson Delatorre 10, Ighor Arantes 11, 12, Marilda Mendonça Siqueira 11, Paola Cristina Resende 11, Tiago Gräf 13 and Gonzalo Bello 12
Affiliations:
1. Laboratório de Ecologia de Doenças Transmissíveis na Amazônia, Instituto Leônidas e Maria Deane, Fiocruz, Manaus, Brazil.
2. Laboratório de Flavivírus, Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, RJ, Brazil.
3. Diretoria, Instituto Leônidas e Maria Deane, Fiocruz, Manaus, Brazil.
4. Fundação Centro de Controle de Oncologia do Estado do Amazonas, Manaus, AM, Brazil.
5. Fundação de Vigilância em Saúde do Amazonas - Dra. Rosemary Costa Pinto, Manaus, AM, Brazil.
6. Laboratório Central de Saúde Pública do Amazonas, Manaus, AM, Brazil.
7. Universidade do Estado do Amazonas, Manaus, AM, Brazil.
8. Hospital Adventista de Manaus, Manaus, AM, Brazil.
9. Departamento de Entomologia e Núcleo de Bioinformática, Instituto Aggeu Magalhães, Fiocruz, Recife, PE, Brazil.
10. Departamento de Biologia. Centro de Ciências Exatas, Naturais e da Saúde, Universidade Federal do Espírito Santo, Alegre, ES, Brazil.
11. Laboratório de Vírus Respiratórios e do Sarampo (LVRS), Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, RJ, Brazil.
12. Laboratório de AIDS e Imunologia Molecular, Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, RJ, Brazil.
13. Instituto Gonçalo Moniz, Fiocruz, Salvador, BA, Brazil.
Summary
The COVID-19 epidemic in the Amazonas state, Brazil, during 2021 was driven by the spread of the Variant of Concern (VOC) Gamma (lineage P.1) and, more recently, of the Gamma plus variants harboring deletions in the N-terminal (NTD) domain (lineage P.1.3) or mutations at the S1/S2 junction (lineages P.1.4 to P.1.6) of the Spike protein. Here, we describe the analysis of 1,132 SARS-CoV-2 whole-genome sequences from individuals diagnosed in the Amazonas state from 1st July to 15th October 2021, representing 4.5% of all confirmed cases in the state in this period. Our genomic survey reveals a sharp reduction in the prevalence of VOCs Gamma and Gamma plus and the increase in the relative prevalence of the VOC Delta from 1% of SARS-CoV-2 samples sequenced in July to 89% of samples in October 2021. During this period, we also detected the introduction of the variant of interest (VOI) Mu, which remained in a low prevalence (<1%) in August and September 2021. These findings revealed that the VOC Delta outcompeted variants Gamma, Gamma plus, and Mu even in the Amazonas State, a setting with one of the world’s highest hybrid (natural and vaccine) immunity thresholds. Notably, while the VOC Delta progressively replaced the other variants between July and October 2021, the total number of SARS-CoV-2 cases decreased in Amazonas.
Text
The Brazilian state of Amazonas was heavily hit by two COVID-19 epidemic waves driven by the persistent circulation of local SARS-CoV-2 lineages and exponential growth of cases. The most long-term persistent SARS-CoV-2 lineage was the B.1.1.28 that circulated in the Amazonas between April 2020 and January 2021. This lineage gave origin to the widely disseminated VOC Gamma (lineage P.1) in late 2020 that fueled the second and largest epidemic wave in the state [1, 2]. By the time the Gamma epidemic was brought under control in March 2021, it was estimated that more than 75% of the population was already infected [3, 4]. Furthermore, vaccination roll-out continuously progressed during 2021, with 61.7% of the individuals in the Amazonas state receiving at least one vaccine dose, and 41.5% were fully vaccinated by 21st October 2021 [5]. This combination produces a high prevalence of individuals with hybrid (natural and vaccine-induced) immunity in this Brazilian state.
Despite the high prevalence of individuals with natural or vaccine-induced immunity in the Amazonas after the second COVID-19 epidemic wave, the virus continues circulating in this state at a roughly steady-state level of ~500 SARS-CoV-2 positive cases per day (7-day rolling average) from early May to mid-July 2021 [6]. This endemic-like pattern of virus transmission was associated with the progressive extinction of the VOC Gamma and the concomitant rise of Gamma plus local variants harboring deletions in the N-terminal (NTD) domain (lineage P.1.3) or, more frequently, mutations at the S1/S2 junction (lineages P.1.4 to P.1.6) of the Spike (S) protein [7]. Gamma plus lineages P.1.4 (P.1+S: N679K) and P.1.6 (P.1+S: P681H) were the most successfully disseminated variants in the Amazonas since March 2021 and combined comprise approximately 90% of all state SARS-CoV-2 positive samples sequenced in the first week of July 2021.
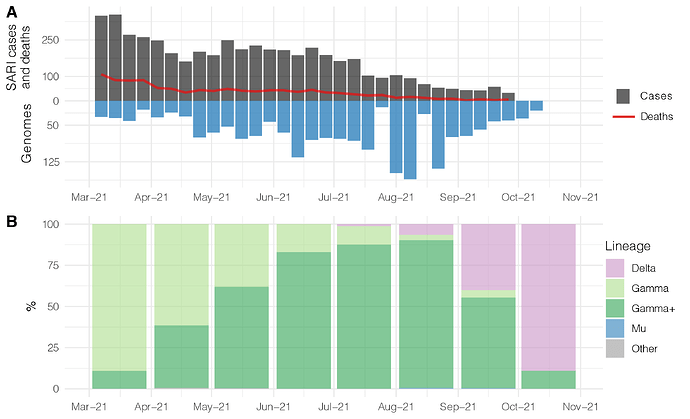
To follow up the evolution of the SARS-CoV-2 in the Amazonas, we sequenced the virus genome from 1,132 patients from 1st July to 15th October 2021, representing 4.5% of all laboratory-confirmed SARS-CoV-2 cases in the state in that period (n = 25,014) (Fig. 1A). The Amazonas state health surveillance foundation sent SARS-CoV-2 positive samples from different municipalities for sequencing at FIOCRUZ Amazônia, part of the local health genomics network (REGESAM) and the consortium FIOCRUZ COVID-19 Genomics Surveillance Network of the Brazilian Ministry of Health [8]. Our genomic survey revealed that Gamma plus (particularly lineages P.1.4 and P.1.6) were the most prevalent variants in the Amazonas until September 2021, when VOC Delta surpassed them. The VOC Delta was first detected in Amazonas on 21st July 2021 and continuously increased in frequency, from 1% in July to 6% in August, 40% in September, and 89% in the first half of October (Fig. 1B). During this period, we also detected for the first time the variant of interest (VOI) Mu at a very low prevalence (<1%) in August and September (Fig. 1B).
Figure 1. Temporal distribution and genetic diversity of SARS-CoV-2-positive samples from the Amazonas state between March and October 2021. A. Graph depicting the temporal evolution of SARI cases and deaths based on the date of symptom onset (source, http://info.gripe.fiocruz.br) as a proxy for the COVID-19 epidemic curve in Amazonas state, along with the number of SARS-CoV-2 whole-genome sequences generated in the study period. B. Relative frequency of different viral variants among SARS-CoV-2 positive cases sequenced in the Amazonas in the study period. The number of SARI cases in October is under review.
Notably, in sharp contrast to the huge COVID-19 epidemic wave associated with the substitution of lineage B.1.1.28 by the VOC Gamma, the most recent viral lineage replacements occurred without a new upsurge of SARS-CoV-2 cases in the Amazonas. During the replacement of Gamma by Gamma plus variants between April and July 2021, the daily number of SARS-CoV-2 cases in the Amazonas remained roughly stable at ~500 cases per day (7-day rolling average) [7]. During the current lineage substitution of Gamma plus by Delta, the 7-day average daily number of SARS-CoV-2 cases dropped from ~500 in mid-July to ~50 in late September and then remained roughly stable until late October 2021 (Fig. 2). We speculate that the high levels of natural and hybrid immunity in the Amazonas state population may have been enough to prevent a new upsurge of SARS-CoV-2 symptomatic cases despite the continuous transmission of more infectious viral variants and the occurrence of successive viral lineage replacements.
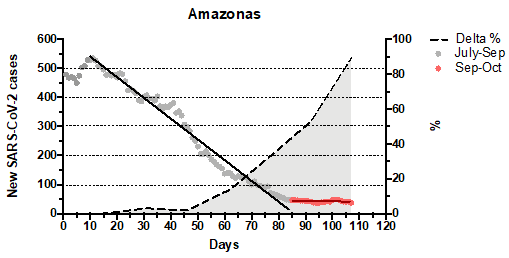
Figure 2. Graph depicting the temporal evolution of daily new SARS-CoV-2 cases (7-day average, source, GitHub - CSSEGISandData/COVID-19: Novel Coronavirus (COVID-19) Cases, provided by JHU CSSE, left y-axis) and the estimated prevalence of Delta variant (right y-axis) in Amazonas state between 1st July and 15th October 2021. Solid lines represent the linear regression analyses of daily new SARS-CoV-2 cases between 10th July and 22nd September 2021 (slope = -7.1, R2 = 0.98, P < 0.0001) and between 23rd September and 15th October 2021 (slope = -0.1, R2 = 0.05, P = 0.3225).
The rapid spread of the VOC Delta in the Amazonas is consistent with the notion that this VOC led to higher viral loads in the upper respiratory tract and is more transmissible than any other SARS-CoV-2 variant recognized to date [9-15]. Interestingly, our genomic survey also detected the introduction of the VOI Mu in the Amazonas, which is one of the most resistant variants to neutralization by sera from convalescent or vaccinated individuals [16, 17]. We may expect that variant Mu could have a selective advantage over other variants in settings with high levels of population immunity. However, we found no evidence that Mu is outcompeting Gamma or Delta in the Amazonas state. Altogether, these findings support the notion that people with hybrid immunity may be able to neutralize most naturally occurring SARS-CoV-2 variants [17-23]. Furthermore, our data suggest that more transmissible variants will most likely drive the endemic transmission of SARS-CoV-2 in highly immunity thresholds settings, rather than variants that may be more competent to evade prior immunity.
In summary, these findings support that after 15 months of persistent transmission and evolution of SARS-CoV-2 (lineage B.1.1.28 and the descendant VOC Gamma and Gamma plus) in the Amazonas state, local variants were displaced by the recently introduced VOC Delta. The high number of individuals with hybrid immunity (naturally infected and vaccinated) in the Amazonas state probably limited the upsurge of cases associated with the spread of SARS-CoV-2 variants that are more transmissible or resistant to neutralization. However, viral lineage replacements driven by more transmissible variants may still occur in these settings. The continuous monitoring of the COVID-19 epidemic in the Amazonas state may provide essential clues to anticipate the future evolution of SARS-CoV-2 in human populations with increasing density of people with hybrid immunity.
Acknowledgments
The authors wish to thank all the health care workers and scientists who have worked hard to deal with this pandemic threat. We also appreciate the support of Genomic Coronavirus Fiocruz Network members and the Respiratory Viruses Genomic Surveillance Network of the General Laboratory Coordination (CGLab) of the Brazilian Ministry of Health (MoH), Brazilian Central Laboratory States (LACENs), and the Amazonas surveillance teams for the partnership in the viral surveillance in Brazil. Funding support FAPEAM (PCTI-EmergeSaude/AM call 005/2020; Rede Genômica de Vigilância em Saúde - REGESAM); Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant 403276/2020-9); Inova Fiocruz/Fundação Oswaldo Cruz (Grant VPPCB-007-FIO-18-2-30 - Geração de conhecimento); Departamento de Ciência e Tecnologia (DECIT) of the Brazilian MoH and OPAS, Brazilian office.
Data availability
All the SARS-CoV-2 genomes generated and analyzed in this study are available at the EpiCoV database in GISAID (https://www.gisaid.org/) and in the **Supplementary table.pdf (121.3 KB)
**.
References
-
Naveca FG, Nascimento V, Souza VCd, Corado AdL, Nascimento F, Silva G, et al. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat Med. 2021;27:1230–8. Epub 25 May 2021. doi: 10.1038/s41591-021-01378-7. PubMed Central PMCID: PMC25 May 2021.
-
Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DDS, Mishra S, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372(6544):815-21. Epub 2021/04/16. doi: 10.1126/science.abh2644. PubMed PMID: 33853970.
-
Buss LF, Prete CA, Jr., Abrahim CMM, Mendrone A, Jr., Salomon T, de Almeida-Neto C, et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371(6526):288-92. Epub 2020/12/10. doi: 10.1126/science.abe9728. PubMed PMID: 33293339; PubMed Central PMCID: PMCPMC7857406.
-
He D, Artzy-Randrup Y, Musa SS, Gräf T, Naveca F, Stone L. The unexpected dynamics of COVID-19 in Manaus, Brazil: Was herd immunity achieved? medRxiv [Internet]. 2021 25 September 2021 25 September 2021]. Available from: The unexpected dynamics of COVID-19 in Manaus, Brazil: Was herd immunity achieved? | medRxiv.
-
Governo do Estado do Amazonas, Fundação de Vigilância em Saúde do Amazonas. Vacinômetro - COVID-19 2021 [cited 2021 03 July]. Available from: Portal FVS-RCP/AM.
-
Governo do Estado do Amazonas, Fundação de Vigilância em Saúde do Amazonas. COVID-19 no Amazonas Dados Epidemiológicos | Boletins e Painéis de Monitoramento de Indicadores 2021 [cited 2021 03 July]. Available from: https://www.fvs.am.gov.br/transparenciacovid19_dadosepidemiologicos.
-
Naveca F, Nascimento V, Souza V, de Lima Corado A, Nascimento F, Silva G, et al. Spread of Gamma (P.1) sub-lineages carrying Spike mutations close to the furin cleavage site and deletions in the N-terminal domain drives ongoing transmission of SARS-CoV-2 in Amazonas, Brazil. medRxiv [Internet]. 2021 2021-01-01 00:00:00. Available from: https://www.medrxiv.org/content/10.1101/2021.09.12.21263453v1.
-
FIOCRUZ. Dashboard Rede Genômica Fiocruz Brazil: FIOCRUZ; 2021 [updated 04 October 202105 October 2021]. Available from: http://www.genomahcov.fiocruz.br/dashboard/.
-
Dhar MS, Marwal R, Vs R, Ponnusamy K, Jolly B, Bhoyar RC, et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. Science. 2021:eabj9932. Epub 2021/10/15. doi: 10.1126/science.abj9932. PubMed PMID: 34648303.
-
Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh M, et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Infect Dis. 2021. Epub 2021/08/24. doi: 10.1093/cid/ciab721. PubMed PMID: 34423834; PubMed Central PMCID: PMCPMC8522361.
-
Luo CH, Morris CP, Sachithanandham J, Amadi A, Gaston D, Li M, et al. Infection with the SARS-CoV-2 Delta Variant is Associated with Higher Infectious Virus Loads Compared to the Alpha Variant in both Unvaccinated and Vaccinated Individuals. medRxiv [Internet]. 2021 2021-01-01 00:00:00. Available from: https://doi.org/10.1101/2021.08.15.21262077.
-
Bolze A, Cirulli ET, Luo S, White S, Wyman D, Rossi AD, et al. SARS-CoV-2 variant Delta rapidly displaced variant Alpha in the United States and led to higher viral loads. medRxiv [Internet]. 2021 2021-01-01 00:00:00. Available from: SARS-CoV-2 variant Delta rapidly displaced variant Alpha in the United States and led to higher viral loads | medRxiv.
-
Li B, Deng A, Li K, Hu Y, Li Z, Xiong Q, et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. medRxiv [Internet]. 2021 2021-01-01 00:00:00. Available from: https://doi.org/10.1101/2021.07.07.21260122.
-
Kang M, Xin H, Yuan J, Ali ST, Liang Z, Zhang J, et al. Transmission dynamics and epidemiological characteristics of Delta variant infections in China. medRxiv [Internet]. 2021 2021-01-01 00:00:00. Available from: Transmission dynamics and epidemiological characteristics of Delta variant infections in China | medRxiv.
-
Earnest R, Uddin R, Matluk N, Renzette N, Siddle KJ, Loreth C, et al. Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA. medRxiv [Internet]. 2021 2021-01-01 00:00:00. Available from: https://doi.org/10.1101/2021.10.06.21264641.
-
Uriu K, Kimura I, Shirakawa K, Takaori-Kondo A, Nakada T-a, Kaneda A, et al. Ineffective neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine sera. bioRxiv [Internet]. 2021 2021-01-01 00:00:00. Available from: Ineffective neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine sera | bioRxiv.
-
Tada T, Zhou H, Dcosta BM, Samanovic MI, Cornelius A, Herati RS, et al. Neutralization of Mu and C.1.2 SARS-CoV-2 Variants by Vaccine-elicited Antibodies in Individuals With and Without Previous History of Infection. bioRxiv [Internet]. 2021 2021-01-01 00:00:00. Available from: Neutralization of Mu and C.1.2 SARS-CoV-2 Variants by Vaccine-elicited Antibodies in Individuals With and Without Previous History of Infection.
-
Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021. Epub 2021/03/27. doi: 10.1126/science.abg9175. PubMed PMID: 33766944; PubMed Central PMCID: PMCPMC8139425.
-
Schmidt F, Weisblum Y, Rutkowska M, Poston D, Da Silva J, Zhang F, et al. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature. 2021. Epub 2021/09/21. doi: 10.1038/s41586-021-04005-0. PubMed PMID: 34544114.
-
Crotty S. Hybrid immunity. Science. 2021;372(6549):1392-3. doi: 10.1126/science.abj2258.
-
Keeton R, Richardson SI, Moyo-Gwete T, Hermanus T, Tincho MB, Benede N, et al. Prior infection with SARS-CoV-2 boosts and broadens Ad26.COV2.S immunogenicity in a variant-dependent manner. Cell Host & Microbe. 2021. doi: 10.1016/j.chom.2021.10.003.
-
Andreano E, Paciello I, Piccini G, Manganaro N, Pileri P, Hyseni I, et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. 2021. Epub 2021/10/21. doi: 10.1038/s41586-021-04117-7. PubMed PMID: 34670266.
-
Callaway E. COVID super-immunity: one of the pandemic’s great puzzles. News [Internet]. 2021 14 October 2021. Available from: COVID super-immunity: one of the pandemic’s great puzzles.