The dissemination of the Omicron variant in the highly seroprevalent Amazonas state, Brazil, is associated with a rapid upsurge of SARS-CoV-2 cases.
Felipe Gomes Naveca 1,2, Valdinete Nascimento 1, Victor Souza 1, Fernanda Nascimento 1, Matilde Mejía 1, Maria Júlia Brandão 1, Arlesson Viana da Silva 1, Dejanane Silva 1, George Silva 3, Luciana Gonçalves 1,4, Tatyana Costa Amorim Ramos 4, Daniel Barros de Castro 4, Tirza Mattos 5, Gabriel Luz Wallau 6, Edson Delatorre 7, Ighor Arantes 8, Marilda Mendonça Siqueira 8, Paola Cristina Resende 8, Tiago Gräf 9 and Gonzalo Bello 10
Affiliations:
1 Laboratório de Ecologia de Doenças Transmissíveis na Amazônia, Instituto Leônidas e Maria Deane, Fiocruz, Manaus, Brazil.
2 Laboratório de Flavivírus, Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, RJ, Brazil.
3 Fundação Centro de Controle de Oncologia do Estado do Amazonas, Manaus, AM, Brazil.
4 Fundação de Vigilância em Saúde do Amazonas - Dra. Rosemary Costa Pinto, Manaus, AM, Brazil.
5 Laboratório Central de Saúde Pública do Amazonas, Manaus, AM, Brazil.
6 Departamento de Entomologia e Núcleo de Bioinformática, Instituto Aggeu Magalhães, Fiocruz, Recife, PE, Brazil.
7 Departamento de Biologia. Centro de Ciências Exatas, Naturais e da Saúde, Universidade Federal do Espírito Santo, Alegre, ES, Brazil.
8 Laboratório de Vírus Respiratórios e do Sarampo (LVRS), Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, RJ, Brazil.
9 Instituto Gonçalo Moniz, Fiocruz, Salvador, BA, Brazil.
10 Laboratório de AIDS e Imunologia Molecular, Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, RJ, Brazil.
Two exponential COVID-19 epidemic waves heavily hit the Brazilian state of Amazonas in early 2020 and early 2021, driven by SARS-CoV-2 variants B.1 (mainly B.1.195 and B.1.1.28) and variant of concern (VOC) Gamma (P.1), respectively [1, 2]. The high prevalence of individuals with hybrid (natural or vaccine-induced) immunity in this Brazilian state [3-5] successfully limited the previous expansion of VOCs Gamma plus (P.1.) [6] and Delta (B.1.617.2/AY.) [7] that emerged and spread during the second half of 2021 in the Amazonas. Consequently, the virus circulated at a roughly steady-state level of ~50-500 SARS-CoV-2 positive cases per day (7-day rolling average) from early May to late December 2021 [8]. In January 2022, however, the mean daily number of SARS-CoV-2 positive cases in the Amazonas rapidly increased from ~90 to ~6,500, coinciding with the global spread of the immune escape VOC Omicron (B.1.1.529/BA.*) [9].
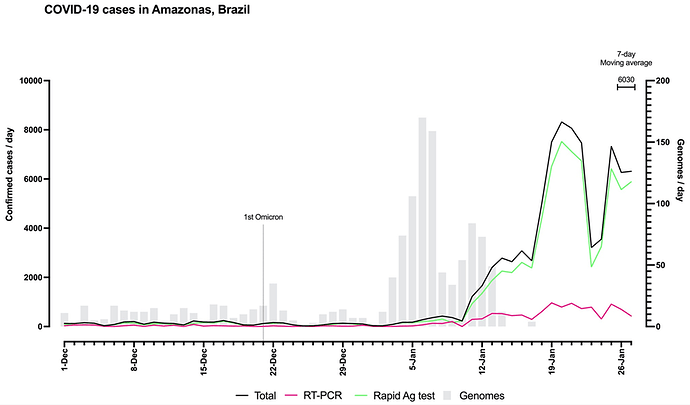
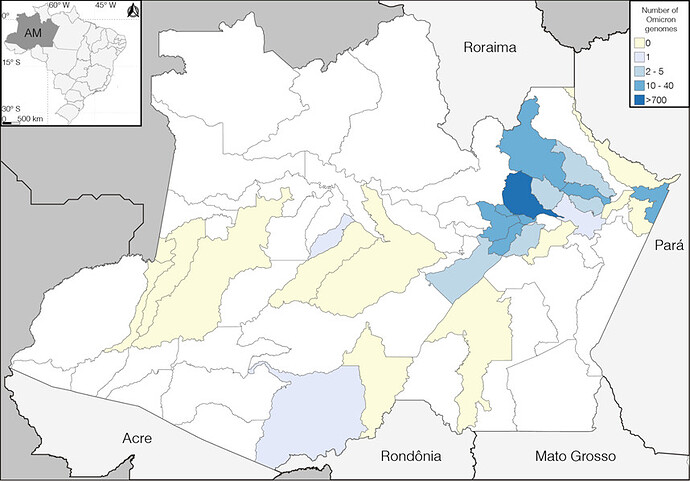
To follow up the most recent evolution of the SARS-CoV-2 in the Amazonas, we sequenced the virus genome from 1,260 patients (707 female, median age 36, IQR 26-47 and 553 male, median age 37, IQR 28-51), collected between 1st December 2021 and 17th January 2022, representing 2.9% of all laboratory-confirmed SARS-CoV-2 cases in the state in that period (n = 43,288) (Fig. 1). The Amazonas state health surveillance foundation and Central Laboratory from the State of Amazonas (LACEN-AM) sent SARS-CoV-2 positive samples from 27 out of 62 municipalities for sequencing at FIOCRUZ Amazônia, part of the local health genomics network (REGESAM) and the consortium FIOCRUZ COVID-19 Genomics Surveillance Network of the Brazilian Ministry of Health [10]. Most samples were from the capital Manaus (n = 926, 74%), followed by the Metropolitan region (n = 163, 13%) and interior municipalities from Central (n = 139, 11%), South (n = 23, 2%) and Southwest (n = 9, 1%) Amazonas regions (Fig. 2).
Genomes were generated using COVIDSeq V3 Primer Pool (Illumina), including other primer sets developed by our team to improve the coverage [6]. Subsequently, libraries were clustered with MiSeq Reagent Kit v3 (600-cycles) on 2 x 150 cycles paired-end runs (Illumina) or with NextSeq 1000 on 2 x 100 cycles. Raw data were converted to FASTQ files at Illumina BaseSpace (https://basespace.illumina.com), and consensus sequences were produced with DRAGEN COVID LINEAGE 3.5.5 or 3.5.6. Genomes were evaluated for mutation calling and quality with Nextclade Web 1.13.0 (https://clades.nextstrain.org) [11]. Sequences with more than 3% of ambiguities “Ns” or any quality flag were reassembled using a customized workflow that employed BBDuk and BBMap tools (v38.84) running on Geneious Prime 2022.0.1. Our consensus sequences had a mean depth coverage higher than 1,800x, excluding duplicate reads. Only those considered as high-quality were considered for further analysis. Whole-genome sequences were classified using the ‘Phylogenetic Assignment of Named Global Outbreak Lineages’ (PANGOLIN) software v3.1.18 (pangolearn 2022-01-20, constellations v0.1.2, scorpio v0.3.16, and pango-designation release v1.2.123) [12].
Figure 1. Graph depicting the temporal evolution of SARS-CoV-2-positive samples (7-day average, source: https://www.fvs.am.gov.br/transparenciacovid19_dadosepidemiologicos) along with the number of SARS-CoV-2 whole-genome sequences here generated from the Amazonas state between December 2021 and January 2022. Lines represent the number of SARS-CoV-2 positive cases detected by rapid antigen test, real-time RT-PCR, or both combined (see color legend). Gray bars represent the number of SARS-CoV-2 genomes sequenced at each sampling day.
Figure 2. Spatial distribution of SARS-CoV-2 sequences sampled in the state of Amazonas between 1st December 2021 and 17th January 2022. Municipalities are colored according to the number of SARS-CoV-2 Omicron samples detected as indicated in the color scale.
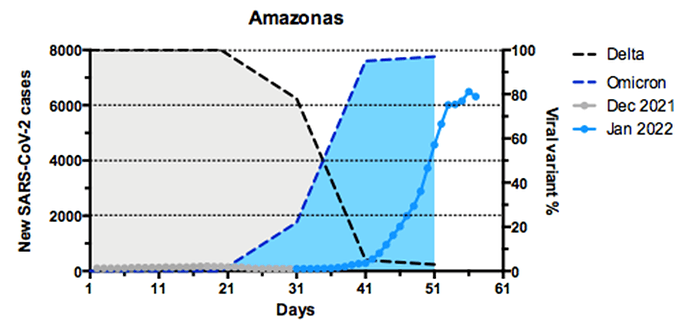
Our genomic survey revealed that Delta was the most prevalent VOC in the Amazonas until late December 2021, but the VOC Omicron rapidly replaced it in January 2022. The VOC Omicron was found in 15 out of 27 municipalities analyzed (Fig. 2). Omicron was first detected in Amazonas on 21st December 2021 in Manaus and rapidly increased in frequency, from 22% in late December 2021 to 95% in early January 2022 (Fig. 3A). There was a lag time of about ten days between the first detection of the VOC Omicron and the beginning of the exponential growth of SARS-CoV-2 cases in Amazonas (Fig. 3A). Outside Manaus, the Omicron variant was first detected on 3rd January in the Metropolitan and Central regions and on 12th January in the South region. The estimated Omicron prevalence in January 2022 in Manaus (98%) and the Metropolitan region (95%) was higher than in interior state regions (60%). Comparative analysis of the temporal prevalence of VOC Omicron in the two most populated cities of the Amazonas state located 372 Km away, the Manaus (capital) and Parintins (Central region), suggests that local dissemination of Omicron probably started in the capital and later spread to inner cities (Fig. 3B).
A
B
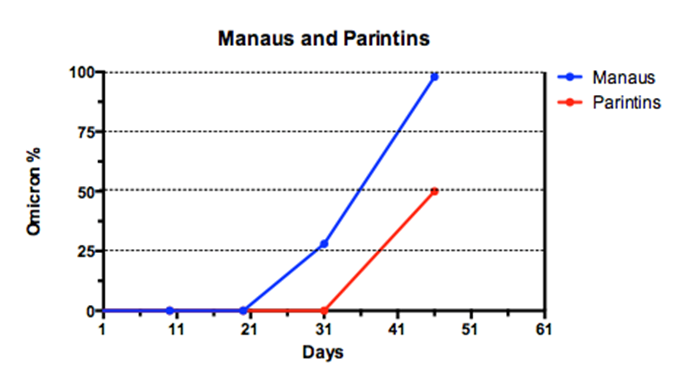
Figure 3. Temporal evolution of prevalence of SARS-CoV-2 VOCs in the Amazonas state between 1st December and 26th January 2022. A) Graph depicting the temporal evolution of daily new SARS-CoV-2 cases (7-day average, source: https://www.fvs.am.gov.br/transparenciacovid19_dadosepidemiologicos, left y-axis) and the estimated prevalence of Delta and Omicron variants (right y-axis) in Amazonas state. B) Graph depicting the temporal evolution of the estimated prevalence of Omicron variant in the two most populated cities of the Amazonas state (Manaus and Parintins).
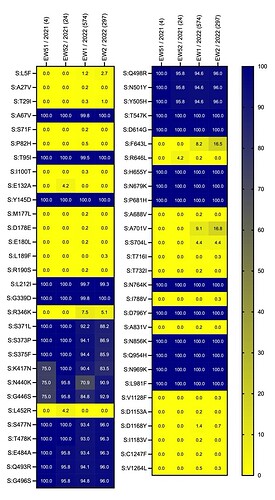
The 903 Omicron sequences from Amazonas were classified within lineages BA.1 (93%) and BA.1.1 (7%, harboring mutation R346K). Mutation analysis at the Spike (S) protein revealed 28 additional acquired mutations among Amazonian sequences (Fig. 4). Most acquired mutations were restricted to one or a few sequences and showed no increase in frequency along consecutive epidemiological weeks (EW), with three exceptions: i) mutation S704L that increased in frequency from 0% in EW 52 to 4% in EW2, and ii) mutations F643L and A701V that increased from 0% in EW 52 to 17% in EW2. It is interesting that mutations F643L and A701V were mapped on the same SARS-CoV-2 genomes, indicating the emergence of a putative new BA.1 sub-lineage in the Amazonas.
Figure 4. The proportion of Amazonian SARS-CoV-2 VOC Omicron sequences containing a given mutation sampled per EW. The proportion of sequences is indicated by the color (according to the scale) and the number within the tile. All mutations with frequencies >70% correspond to Omicron synapomorphies. The total number of sequences per EW is indicated in parentheses.
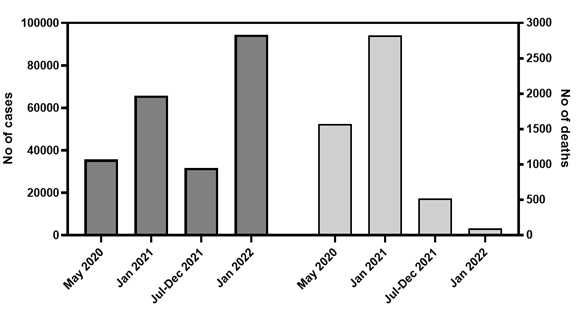
These findings confirm that the SARS-CoV-2 Omicron variant is driving the third COVID-19 epidemic wave in Amazonas. Omicron replaced Delta as the most prevalent SARS-CoV-2 variant in the capital Manaus and the Metropolitan region in only three weeks, between mid-December 2021 and early January 2022, and the same pattern is being observed in the inner state municipalities. The high level of hybrid immunity in the Amazonas state limited the upsurge of cases associated with the spread of highly transmissible variants like Gamma plus and Delta. However, it could not prevent a new expansion of SARS-CoV-2 cases associated with the spread of the Omicron variant that combined both enhanced transmissibility and substantial immune escape [13-16]. Despite the record number of SARS-CoV-2 positive cases registered in Amazonas in January 2022, the proportion of deaths during the Omicron wave is currently much lower than that registered during previous epidemic waves or the previous endemic-like period (Fig. 5). This observation is consistent with the notion that acquired immunity (natural plus vaccines) effectively reduces the infection fatality rate of SARS-CoV-2 in Amazonas over time.
A
B
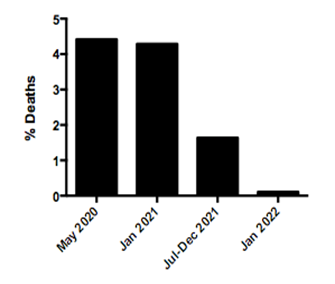
Figure 5. Temporal evolution of SARS-CoV-2-positive cases and deaths in the Amazonas state during the exponential epidemic waves of B.1 lineage (May 2020), VOC Gamma (January 2021), VOC Omicron (January 2022), and during the endemic-like period of circulation of VOCs Gamma plus and Delta (July to December 2021) (source: https://www.fvs.am.gov.br/transparenciacovid19_dadosepidemiologicos). A) Graphs depicting the total number of SARS-CoV-2-positive cases (left y-axis, dark gray bars) and deaths (right y-axis, light gray bars) at the different time periods. B) Graph depicting the proportion of total deaths relative to the total number of SARS-CoV-2-positive cases at the different time periods.
Acknowledgments
The authors wish to thank all the health care workers and scientists who have worked hard to deal with this pandemic threat. We also appreciate the support of FIOCRUZ COVID-19 Genomics Surveillance Network members and the Respiratory Viruses Genomic Surveillance Network of the General Laboratory Coordination (CGLab) of the Brazilian Ministry of Health (MoH), Brazilian Central Laboratory States (LACENs), and the Amazonas surveillance teams for the partnership in the viral surveillance in Brazil. Funding support FAPEAM (PCTI-EmergeSaude/AM call 005/2020; Rede Genômica de Vigilância em Saúde - REGESAM); Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant 403276/2020-9); Inova Fiocruz/Fundação Oswaldo Cruz (Grant VPPCB-007-FIO-18-2-30 - Geração de conhecimento); Centers for Disease Control and Prevention (CDC Grant Award 002174); Departamento de Ciência e Tecnologia (DECIT) of the Brazilian MoH and OPAS, Brazilian office.
Data availability
All the SARS-CoV-2 genomes generated and analyzed in this study are available at the EpiCoV database in GISAID (https://www.gisaid.org/) and in the Supplementary table (Supplementary.pdf (840.9 KB)).
References
-
Faria, N.R., et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science, 2021. 372, 815-821 DOI: 10.1126/science.abh2644.
-
Naveca, F.G., et al. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat Med, 2021. 27, 1230–1238 DOI: 10.1038/s41591-021-01378-7.
-
Buss, L.F., et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science, 2021. 371, 288-292 DOI: 10.1126/science.abe9728.
-
He, D., et al. The unexpected dynamics of COVID-19 in Manaus, Brazil: Was herd immunity achieved? medRxiv, 2021. DOI: The unexpected dynamics of COVID-19 in Manaus, Brazil: Was herd immunity achieved? | medRxiv.
-
Governo do Estado do Amazonas and Fundação de Vigilância em Saúde do Amazonas. Vacinômetro - COVID-19. 2021 [cited 2021 03 July]; Available from: Portal FVS-RCP/AM.
-
Naveca, F., et al. Spread of Gamma (P.1) sub-lineages carrying Spike mutations close to the furin cleavage site and deletions in the N-terminal domain drives ongoing transmission of SARS-CoV-2 in Amazonas, Brazil. medRxiv, 2021. DOI: 10.1101/2021.09.12.21263453.
-
Naveca, F.G., et al. The SARS-CoV-2 variant Delta displaced the variants Gamma and Gamma plus in Amazonas, Brazil. Virological.org 2021; Available from: https://pando.tools/t/the-sars-cov-2-variant-delta-displaced-the-variants-gamma-and-gamma-plus-in-amazonas-brazil/765.
-
Governo do Estado do Amazonas and Fundação de Vigilância em Saúde do Amazonas. COVID-19 no Amazonas Dados Epidemiológicos | Boletins e Painéis de Monitoramento de Indicadores. 2021 [cited 2021 03 July]; Available from: https://www.fvs.am.gov.br/transparenciacovid19_dadosepidemiologicos.
-
Viana, R., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature, 2022. DOI: 10.1038/s41586-022-04411-y.
-
FIOCRUZ. Dashboard Rede Genômica Fiocruz. 2021 04 October 2021 05 October 2021]; Available from: http://www.genomahcov.fiocruz.br/dashboard/.
-
Aksamentov, I., et al. Nextclade: clade assignment, mutation calling and quality control for viral genomes. Journal of Open Source Software, 2021. 6, 3773 DOI:10.21105/joss.03773.
-
Rambaut, A., et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol, 2020. DOI: 10.1038/s41564-020-0770-5.
-
Lyngse, F.P., et al. SARS-CoV-2 Omicron VOC Transmission in Danish Households. medRxiv, 2021. DOI: 10.1101/2021.12.27.21268278.
-
Chaguza, C., et al. Rapid emergence of SARS-CoV-2 Omicron variant is associated with an infection advantage over Delta in vaccinated persons. medRxiv, 2022. DOI: 10.1101/2022.01.22.22269660.
-
Peacock, T.P., et al. The SARS-CoV-2 variant, Omicron, shows rapid replication in human primary nasal epithelial cultures and efficiently uses the endosomal route of entry. bioRxiv, 2022. DOI: 10.1101/2021.12.31.474653.
-
Ravindra, G. SARS-CoV-2 Omicron spike mediated immune escape and tropism shift. Nature Portfolio, 2022. DOI: 10.21203/rs.3.rs-1191837/v1.