First 2022 monkeypox outbreak genome sequences from Slovenia
Tomaž Mark Zorec ¹, Samo Zakotnik ¹, Alen Suljič ¹, Doroteja Vlaj ¹, Maja Mastnak ², Damjana Rozman ³, Cene Skubic ³, Miša Korva ¹, Tatjana Avšič-Županc ¹* and Mario Poljak ¹*
¹ Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, Slovenia
² Department for sexually transmitted diseases of Dermatovenerologic Clinic, University Medical Centre Ljubljana, Slovenia
³ Centre for Functional Genomics and Bio-Chips, Institute of Biochemistry, Faculty of Medicine, University of Ljubljana, Slovenia
*contact: [email protected], [email protected]
Monkeypox virus (MPXV) is an important zoonotic pathogen which causes a smallpox-like disease. Two phylogenetic clades of MPXV have been characterized in the past, the central African MPXV endemic to Congo Basin, and the west African MPXV endemic to West Africa. The two genotypes have been associated with varying disease severities and mortality rates: the western type has been shown to cause milder disease and a lower mortality rate (Durski et al., 2018). Although endemic to Sub-Saharan Africa, outbreaks outside of Africa linked to travelers returning from endemic regions have been reported in the USA, the UK, and elsewhere (Hutson et al., 2007; Zumla et al., 2022; WHO, 2022).
Since May 7th , 2022, several cases of monkeypox have been reported in various countries worldwide, including the UK, the USA, Canada, Australia, and many countries of the European Union (Zumla et al., 2022). Based on current data the West African MPXV genotype is central to the ongoing outbreak (Isidro et al., 2022).
Here we announce two complete MPXV genome sequences obtained in Slovenia, related to the recent worldwide outbreak.
The two cases of monkeypox were identified in Slovenia on May 23rd and 25th 2022. Both patients were examined at the Department for sexually transmitted diseases of Dermatovenerologic Clinic, University Medical Centre Ljubljana, after returning from the Canary Islands, Spain, due to acute symptoms of orthopoxvirus infection, including general malaise, swollen inguinal nodes, and anogenital skin lesions. Perianal swab samples and bioptic samples of perianaly distributed papules were collected in UTM Viral Transport Media and tested positive for orthopoxviruses using 2 different real-time PCR assays (Schroeder et al. 2010 and RealStar Orthopoxvirus PCR Kit RUO, Altona, Hamburg, Germany). Monkeypox virus (MPXV), specifically the West African genetic lineage, was confirmed with real-time PCR assays for the specific detection of MPXV (Li et al. 2010). An initial draft genome sequence was obtained based on nanopore long read maps of sample 1. Long reads were sequenced using an ONT GridION instrument with adaptive sequencing. The GenBank sequence MT903344.1 was used as reference. Long-read mapping was performed using minimap2 (v2.20-r1061) (Li, 2018), samtools (v1.9) (Li et al., 2009) and iVar (v1.0) (Grubaugh et al., 2019). Further refinement was performed using medaka (v1.5.0) (Lee et al., 2021). Illumina reads (2 × 150 bp; 20 M paired reads / sample) were sequestered for each sample using an Illumina NextSeq550 instrument and mapped to the draft genome sequence using bwa mem v0.7.17-r1188 (Li, 2013). The consensus sequences were produced using iVar and refined using pilon v1.23 (Walker et al., 2014). Draft functional annotations were generated using Prokka v1.14.5 (Seeman, 2014). The complete MPXV genome sequences have been submitted to GenBank and are available under the accession numbers ON609725.2 (sample 1) and ON631241.1 (sample 2); the novel sequences were covered 2380× and 4018×, respectively, on average.
Background and 2022-outbreak complete genome MPXV sequences were downloaded from GenBank and virological.org. A complete genome multiple sequence alignment was produced using mafft (Katoh et al., 2013) and a phylogenetic tree (Figure) was inferred using iqtree2 (Minh et al., 2020) with the substitution model GTR+F+R4 and node support values were estimated using UFBootstrap (Hoang et al., 2018). The Slovenian sequences positioned well within the 2022 outbreak phylogenetic cluster, confirming that the cases are molecularly related. Unexpectedly, the first Belgian 2022 outbreak MPXV genome sequence (ITM-MPX-1-Belgium-2022) did not group immediately alongside the other outbreak sequences, but deeper alongside sequences dating back to 2020. Perhaps this was due to bias related to keeping reference sequence nucleotides at regions insufficiently covered by sample sequence reads during consensus calls.
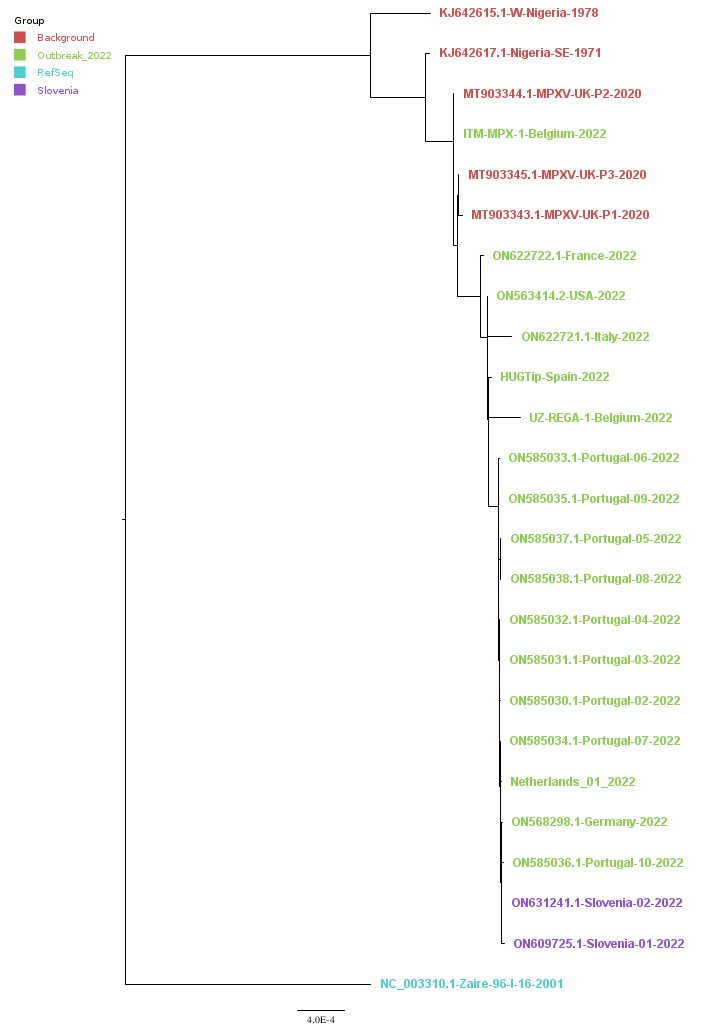
Figure. Phylogenetic tree of background and 2022 outbreak MPXV genome sequences.
Acknowledgements:
This work was supported by the Slovenian Research Agency programmes P3-0083 and P1-0390, by ELIXIR-SI-RI-SI-2, and by MRIC-ELIXIR.
References:
Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004 Jan;4(1):15-25. https://doi.org/10.1016/s1473-3099(03)00856-9 Erratum in: Lancet Infect Dis. 2004 Apr;4(4):251. PMID: 14720564.
Durski KN, McCollum AM, Nakazawa Y, Petersen BW, Reynolds MG, Briand S, Djingarey MH, Olson V, Damon IK, Khalakdina A. Emergence of Monkeypox - West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018 Mar 16;67(10):306-310. https://doi.org/10.15585/mmwr.mm6710a5 Erratum in: MMWR Morb Mortal Wkly Rep. 2018 Apr 27;67(16):479. PMID: 29543790; PMCID: PMC5857192.
Hutson CL, Lee KN, Abel J, Carroll DS, Montgomery JM, Olson VA, Li Y, Davidson W, Hughes C, Dillon M, Spurlock P, Kazmierczak JJ, Austin C, Miser L, Sorhage FE, Howell J, Davis JP, Reynolds MG, Braden Z, Karem KL, Damon IK, Regnery RL. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007 Apr;76(4):757-68. PMID: 17426184.
Zumla A, Valdoleiros SR, Haider N, Asogun D, Ntoumi F, Petersen E, Kock R. 2022. Monkeypox outbreaks outside endemic regions: scientific and social priorities. The Lancet Infectious Diseases, ARTICLE IN PRESS. https://doi.org/10.1016/S1473-3099(22)00354-1
World Health Organization (WHO), 2022. Multi-country monkeypox outbreak in non-endemic countries. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 Accessed 31.3.2022.
Isidro J, Borges V, Pinto M, Ferreira R, Sobral D, Nunes A, Dourado Santos J, Mixão V, Santos D, Duarte S, Vieira L, Borrego MJ, Núncio S, Pelerito A, Cordeiro R, Gomes JP AND Discussion Thread. Multi-country outbreak of Monkeypox virus: genetic divergence and first signs of microevolution. Virological.org https://pando.tools/t/multi-country-outbreak-of-monkeypox-virus-genetic-divergence-and-first-signs-of-microevolution/806
Schroeder K., Nitsche A. 2010. Multicolour, multiplex real-time PCR assay for the detection of human-pathogenic poxviruses. Molecular and Cellular Probes, 24: 110-113.
Li Y, Zhao H, Wilkins K, Hughes C, Damon IK (2010) Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods 169:223–227.
Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018 Sep 15;34(18):3094-3100. https://doi.org/10.1093/bioinformatics/bty191 PMID: 29750242; PMCID: PMC6137996.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009 Aug 15;25(16):2078-9. https://doi.org/10.1093/bioinformatics/btp352 Epub 2009 Jun 8. PMID: 19505943; PMCID: PMC2723002.
Grubaugh ND, Gangavarapu K, Quick J, Matteson NL, De Jesus JG, Main BJ, Tan AL, Paul LM, Brackney DE, Grewal S, Gurfield N, Van Rompay KKA, Isern S, Michael SF, Coffey LL, Loman NJ, Andersen KG. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 2019 Jan 8;20(1):8. https://doi.org/10.1186/s13059-018-1618-7 PMID: 30621750; PMCID: PMC6325816.
Li H. (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:1303.3997v2
Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, Earl AM. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014 Nov 19;9(11):e112963. https://doi.org/10.1371/journal.pone.0112963 PMID: 25409509; PMCID: PMC4237348.
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. 2020. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era, Molecular Biology and Evolution. 37(5). 1530–1534. https://doi.org/10.1093/molbev/msaa015
Lee, J.Y., Kong, M., Oh, J. et al. Comparative evaluation of Nanopore polishing tools for microbial genome assembly and polishing strategies for downstream analysis. Sci Rep 11, 20740 (2021). https://doi.org/10.1038/s41598-021-00178-w
Seemann, T. Prokka: rapid prokaryotic genome annotation, Bioinformatics, Volume 30, Issue 14, 15 July 2014, Pages 2068–2069, https://doi.org/10.1093/bioinformatics/btu153
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013 Apr;30(4):772-80. https://10.1093/molbev/mst010 Epub 2013 Jan 16. PMID: 23329690; PMCID: PMC3603318.
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol Biol Evol. 2020 May 1;37(5):1530-1534. https://10.1093/molbev/msaa015 Erratum in: Mol Biol Evol. 2020 Aug 1;37(8):2461. PMID: 32011700; PMCID: PMC7182206.
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol Biol Evol. 2018 Feb 1;35(2):518-522. https://10.1093/molbev/msx281 PMID: 29077904; PMCID: PMC5850222.