SARS-CoV-2 Spike displays multiple adaptive changes in addition to the furin cleavage site.
Robert F. Garry1-3
1Department of Microbiology and Immunology, Tulane University Medical Center, 1430 Tulane Avenue, New Orleans, Louisiana 70112 USA.
2Zalgen Labs, LLC, Germantown, MD, USA.
3Global Viral Network, Tampa, Florida
Abstract
SARS-CoV-2 is a pandemic pathogen with highly transmissibility by the respiratory route and ability to replicate in many animals, including humans. The evolution of SARS-Cov-2 from bat sarbecoviruses involved multiple changes in Spike in addition to insertion of the furin cleavage site.
Introduction
Spike, the coronavirus surface glycoprotein, is a class I viral fusion protein that mediates attachment, membrane fusion and entry. It is present in virions as a trimer of S1/S2 heterodimers. In the case of SARS-CoV-2 an out-of-frame insertion of 12 nucleotides in the Spike gene added 4 amino acids (proline [P], arginine [R], arginine [R] and alanine [A]) creating a functional polybasic (furin) cleavage site (FCS) at the junction of the S1 and S2 subunits (1, 2). Although some bat sarbecoviruses such as RmYN02 have incomplete FCS (3), to date SARS-CoV-2 is the only known member of the sarbecovirus subgenus of the genus Betacoronaviridae whose Spike has a complete FCS at the S1/S2 junction. The FCS is an important determinant of SARS-CoV-2 transmission and pathogenesis (4-6). Construction of SARS-CoV-2 loss-of-function variants lacking the FCS site (SC2ΔFCS or ΔPRRA) have reduced pathogenicity and transmissibility in animal models (4, 6, 7). However, the FCS in Spike is not the only feature that enabled SARS-CoV-2 to cause the massive sustained COVID-19 pandemic.
Numerous close relatives of SARS-CoV-2 have been detected in Rhinolophus [horseshoe] bats, indicating that these animals are the natural hosts of the ancestors of SARS-CoV-2 (8). The SARS-CoV-2 genome is a mosaic of several distinct sarbecoviruses that have been assembled by recombination, a process that is commonly observed in coronavirus evolution (8, 9). BANAL‐20-52 was sequenced from fecal swabs collected in 2020 from Rhinolophus malayanus in the Lao People’s Democratic Republic (9). The BANAL‐20-52 genome with 96.8% nucleotide sequence identity is the closest overall to the SARS-CoV-2 genome to date. Other sarbecoviruses show greater similarity in some gene segments BANAL-20-52 confirming the mosaic derivation of the SARS-CoV-2 genome.
Hu-1, the prototype strain of SARS-CoV-2, was isolated from a purchaser who traded at the Huanan Market, the likely site of the first spillovers of SARS-CoV-2 or its direct progenitor from non-bat animals to humans (10). BANAL‐20-52 and a related sarbecovirus BANAL-20-236 bind the cell surface receptor human angiotensin‐converting enzyme 2 (hACE2) more efficiently than Hu-1 (9). However, the ability to bind to non-bat ACE2s is not sufficient for human transmission and pathogenesis. BANAL-20-52 and -236 have impaired replication in human nasal epithelial cells and in the upper airway of mice (7, 11). Furthermore, reduced replication of these viruses was observed in the lungs of mice. They were less pathogenic in mice and diminished transmission was observed in hamsters. BANAL-20-236 exhibits tropism toward intestinal cells rather than respiratory cells (11). In macaques BANAL-20-236 displayed enteric tropism whereas SARS‐CoV‐2 showed lung tropism and respiratory shedding (12).
All trimeric Spike proteins of bat sarbecoviruses structurally analyzed to date including BANAL-20-52 and -236 have been shown adopt a predominantly “closed” conformation (13-15). In the closed conformation all three carboxyl terminal domains (CTD) of each monomer in the trimer are in the down position. The CTD contains the receptor binding domain (RBD), which must be in the up position to effectively bind ACE2. Bat sarbecovirus Spike trimers are more compact with additional close packing in various domains, including Domain D, than SARS-CoV-2 Spike trimers (14). A consequence of the tight packing is that BANAL-20-52 and -236 Spikes are more resistant to trypsin digestion at pH 5.5, a condition roughly mimicking conditions in the bat’s stomach (13). While the open conformation of SARS-CoV-2 Spike with one or more RBD in the up position enhances ACE2 binding, the virion becomes more sensitive to inactivation by low pH.
Considering the mosaic genome of SARS-CoV-2 and the switch from gastrointestinal to respiratory transmission in non-bat animals during SARS-CoV-2 evolution, it is evident that BANAL‐20-52 itself is not the direct progenitor of SARS-CoV-2. However, comparison of BANAL‐20-52 and related bat sarbecoviruses with early SARS-Cov-2 isolates can reveal adaptive changes in addition to the FCS that were acquired by SARS-CoV-2 progenitors.
Methods
Spike protein amino acid sequences or spike gene sequences for SARS-CoV-2 Wuhan-Hu-1 (QHD43416.1), Bat coronaviruses: BANAL-20-52 (UAY13217.1), BANAL-20-236 (UAY13253.1), BtSY2 (WBV74286.1), Rp22DB159 (WLJ60537.1), RatG13 (QHR63300.2), RmYN02 (ESL_ISL_ 852605), WIV16 (ALK02457), Pangolin coronavirus isolate MP789 (QIG55945.1) and SARS-CoV strain Urbani (AYV99817.1) were aligned using Clustal Omega (16) or Lalign (17).
The Cryo-EM structure of BANAL-20-52 spike protein PDB8XYH (unpublished results by Xu ZP, Li LJ, Gu YH, Qi JX and Gao GF) was rendered using the Pymol Molecular Graphics System v. 2.5.4 from Schrödinger, LLC.
Results
Comparison of BANAL-20-52 Spike to SAR-CoV-2 Spike
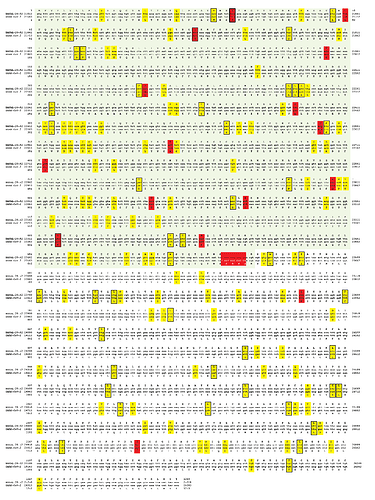
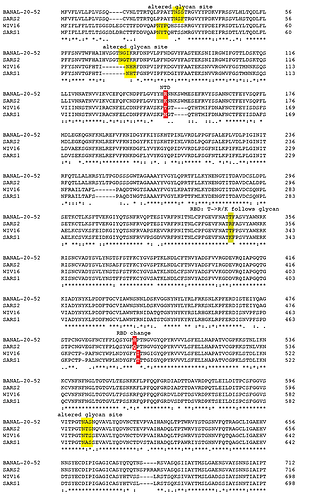
BANAL-20-52 (Laos) Spike shares 98.4% amino acid sequence identity with SARS-CoV-2 Hu-1 (Wuhan, China) Spike; 99.2% are chemically similar amino acids (Fig. 1). Other sarbecoviruses with high amino acid sequence similarity to SARS-CoV-2 that have been detected in bats from the karst terrain common to China, Laos and Vietnam include BtSy2 (Yunnan province, China) 98% identical/99.3% similar, Rp22DB159 (Vietnam) 97.9% identical/99.4% similar and RaTG13 (Yunnan province, China) 97.4% identity (98.7% similar). The S1 subunit of BANAL-20-52 Spike has 14 amino acid differences and the 4 amino acid FCS insertion compared to SARS-CoV-2 Spike S1. The amino-terminal domain (NTD) is 98.1% identical and the carboxyl terminal domain (CTD) that includes receptor binding domain (RBD) is 97.4% identical. There are 2 amino acid differences between the S2 subunits of BANAL-20-52 and SARS-CoV-2 Hu-1.
In contrast to the limited number of amino acids (16) that differ between BANAL-20-52 and SARS-CoV-2 Hu-1 Spike there are considerably more differences at the nucleotide level (Fig. 1). There are 138 synonymous changes between the BANAL-20-52 and SARS-CoV-2 Spike genes. In S1 there are 76 synonymous changes and in S2 there are 62. Codon usage from a bat virus related to BANAL-20-52 drifted or was optimized as the virus adapted to a non-bat host(s).
Figure 1. Comparison of BANAL-20-52 Spike to SAR-CoV-2 Spike. Red box: nonsynonymous; yellow box: synonymous; box with black outline; C→U.
Figure 2. Annotation of the BANAL-20-52 Spike structure highlighting different amino acids from SARS-CoV-2 Hu-1 Spike.
There is a bias for cytosine to uracil (C → U) changes (Fig. 1). 23 of 76 and 23 of 62 synonymous or nonsynymous changes and in S1 and S2 respectively are C → U. This hypermutation phenomenon is host-derived rather than being an intrinsic property of sarbecoviruses. Simmonds (18) suggested that sarbecovirus replication in bats is not associated with C→U hypermutation as found during SARS-CoV-2 replication in humans or other mammals, notably white-tailed deer…
Amino acid changes in the amino terminal domain
Two amino acids in the NTD differ between BANAL-20-52 and SARS-CoV-2 Hu-1 (I76T and R147K) (Fig. 2).
I76T adds a glycan at N74, which may decrease exposure of some NTD epitopes.
R147K is a conservative amino acid change that is unlikely to have a major impact on NTD function or immunogenicity.
Amino acid changes in the carboxyl terminal domain
Four amino acids in the CTD differ between BANAL-20-52 and SARS-CoV-2 Hu-1 (T372A, T346R and H498Q) (Fig. 2).
T346R in the RBD interacts directly with ACE2. T346R in combination with other changes may broaden the receptor usage from bat ACE2 to include ACE2 of other mammals (19).
T372A removes the glycan at N370. All sarbecoviruses, except SARS-CoV-2, including those from bats and pangolins, have N370 and T372 (20). The glycan at N370 crosses over and interacts via hydrogen bonding with neighboring monomers (21). These interactions favor the closed conformation of Spike. The T372A change in SARS-CoV-2 that removes the glycan at N370 facilitates the open conformation of Spike, increases binding to ACE2 and enhances infectivity (13). This mutation is also important for increasing the pH sensitivity of SARS-CoV-2 Spike.
H498Q is in the RBD and interacts directly with ACE2. H498Q reduces infectivity in the cells expressing Rhinolophus bat ACE2 (13). Q498H in SARS-CoV-2 Spike markedly increases pseudoviral entry on R. malayanus, R. sinicus, and P. abramus ACE2. Conversely, a H498Q change in BANAL-20-52 Spike led to a markedly reduced efficiency of virus entry via three bat ACE2s (13). This residue is variable in bat sarbecoviruses. RatG13 Y498 and modifying this residue decrease affinity for bat ACE2 (22).
V402I This conservative change may have little impact on structure or function of Spike.
Amino acid changes in the hinge regions.
Six amino acids differ between BANAL-20-52 and SARS-CoV-2 Hu-1 Spikes in regions that can be thought of as the “hinges” (NTD hinge: S32F, L50S, P218Q and S292A, CTD hinge: H519N, I534V) (Fig. 2). These amino acids are in sequences that link the NTD or CTD to the core of Spike.
S32F removes a glycan at N30. The removal of the glycan may alter exposure of NTD epitopes. Because of the location of this glycan near domain D it may also affect the transition between the closed and open conformations of the Spike trimer (Fig. 2).
L50S is in the vicinity of domain D and may affect transitioning between the closed and open conformations of the Spike trimer.
P218Q in Hu-1 may lead to a more flexible hinge facilitating movement of the NTD. The presence of proline in the bat sarbecovirus Spike could render the hinge more rigid affecting transitioning between the closed and open conformations of the Spike trimer.
S292A may affect transitioning between the closed and open conformations of the Spike trimer.
N519H is positioned in the CTD hinge region and also in close proximity to the adjacent monomer of the trimer. H519 may increase up positioning of CTD facilitating ACE2 binding (23). Pseudotyped viruses bearing N519 demonstrated reduced infectivity in cells expressing the human ACE2 receptor compared to H519.
I534V is a conservative change in the hinge region that is likely to have only a modest influence on up and down transition of the CTD, if any.
Amino acid changes in Domain D
Domain D assumes distinct conformations in bat sarbecovirus Spikes compared to SARS-CoV-2 Spike (14). Domain D contains the FCS a structure referred to as the 630 loop. The 630 loop is located in proximity of the S1/S2 boundary. The packing of the 630 loop influences the propensity of the Spike trimer to change from the closed to open conformations. Two amino acids in Domain D differ between BANAL-20-52 and SARS-CoV-2 Hu-1 (A604T, N627D) (Fig. 2)
A604T retains the glycan at N603 and is unlikely to have has a major influence on Spike structure or function.
N627D is present in the 630 loop suggesting that it could affect the conformation of Domain D and packing of the Spike trimer.
Similar adaptations occurred in the bat sarbecovirus progenitors of SARS-CoV.
Several bat sarbecoviruses have high sequence similarity to SARS-CoV (8). Bat sarbecovirus WIV16, sequenced from a fecal sample of Rhinolophus sinicus collected in Yunnan Province in 2013, has 96.9% identity (98.5% similar) at the amino acid level to the prototype Urbani strain of SARS-CoV (24). Alignment of SARS-CoV Urbani Spike with WIV16 Spike reveals several amino acid differences in locations that are similar to the locations of amino acid differences in SARS-CoV-2 Spike and BANAL-20-52 Spike (Fig. 3). There is an addition of a glycan at SARS-CoV Spike N29 due to a P31T change. This change mirrors the removal of the BANAL-20-52 Spike glycan at N30 due to S32F. There are additions of glycans at the same locations in SARS-CoV Spike by R76T and in SARS-CoV-2 Spike by I76T. Only two amino acids T346R and H498Q differ between the RBDs of SARS-CoV-2 and BANAL-20-52. Notably, the alignment of SARS-CoV and SARS-CoV-2 shows that residues in similar locations also differ between WIV16 and SARS-CoV. SARS-CoV T333K is at the analogous position as SARS-CoV-2 T346R following a conserved glycan. SARS-CoV Spike I486T is one reside different from the analogous location of SARS-CoV-2 Spike H498Q. SARS-CoV Spike differs from WIV16 Spike at two addition amino acids T144M and T590A that also differ in analogous locations between SARS-CoV-2 Spike and BANAL-20-52 Spike
Figure 3. Amino acid alignment of the Spikes of SARS-CoV, SARS-CoV-2 and closely related Spikes of bat sarbecoviruses BANAL-20-52 and WIV16.
Conclusions
SARS-CoV-2 did not suddenly appear pre-adapted for human transmission and pathogenesis. The evolutionary process that resulted in SARS-CoV-2 becoming a human pandemic virus involved adaption of ancestral sarbecoviruses for replication in non-bats hosts and for spread by the respiratory route. There were multiple adaptive changes in addition to the FCS. Comparisons of the Spike of BANAL-20-52, which is the closest to SARS-CoV-2-Hu-1 Spike at the amino acid level, reveals key adaptations beyond the FCS that likely occurred prior to the spillover of SARS-CoV-2’s immediate progenitor to humans. These changes include the:
• T372A change in the CTD that removes the glycan at N370 and facilitates the open, less compact conformation of Spike (21). The resulting higher propensity to present CTDs with the RBD in the up increases binding to ACE2 and enhances infectivity. The more open conformation of Spike is also more sensitive to low pH a finding consistent with a preference for replication in the respiratory tract rather than the gastrointestinal tract.
• N519H change in the CTD hinge region that also facilitates conversion from the closed to open configuration of Spike (23).
• RBD change T346R that contributes to increased binding to the ACE2 of non-bat animals (19) and the other RBD change H498Q that decreases binding to bat ACE2 (13).
• S32F change that removes a glycan and the I76T change that adds a glycan significantly remodeling the Spike glycan shield.
It is highly unlikely that any of these changes occurred during circulation of a SARS-CoV-2 progenitor in a bat. T372A and N519 make the Spike protein more susceptible to low pH degradation and therefore these changes are not compatible with the gastrointestinal tropism of bat sarbecoviruses. H498Q directly reduces infectivity in cells expressing Rhinolophus ACE2 and therefore could not have been selected for during bat transmission. After SARS-CoV-2 spilled over to human the Spike of the dominant lineage B viruses all acquired a D614G change that decreases an important interaction between monomers further promoted opening of the Spike trimer (25, 26). It is notable that certain of these changes, including alterations to key residues in the RBD and remodeling of the glycan shield, have analogs in evolution of SARS-CoV from its bat progenitors.
It is very unlikely that acquisition of a FCS occurred during sarbecovirus transmission among bats. While FCS are common in Spike proteins of coronaviruses (27) and some sarbecoviruses have insertions that form incomplete FCSs (3), no bat sarbecovirus yet sequenced has been shown to have a complete FCS at the S1/S2 junction. Cleavage at the FCS opens up the conformation of SARS-CoV-2 Spike (28), a process that would not be favored in the low pH environment of the gastrointestinal tract. Based on available evidence, the FCS likely arose in a SARS-CoV-2 progenitor during respiratory tract transmission and replication in a non-bat animal.
Table 1. Polybasic cleavage sites in current circulating strains of H5N1 influenza virus
Clade Accession(GSAID) Designation Sequence at HA1/HA2 junction (aa321-360)
2.3.4.4b B3.13 EPI_ISL_19590708 A/California/2024 321 YVKSNKLVLA TGLRNNPLRE KRRKRGLFGA IAGFIEGGWQ 360
2.3.4.4b D1.1 EPI_ISL_19634828 A/Louisiana/2024 321 YVKSNKLVLA TGLRNSPLRE RRRKRGLFGA IAGFIEGGWQ 360
2.3.4.4b D1.3 EPI_ISL_19785793 A/Ohio/2025 321 YVKSNKLVLA TGLRNSPLRE RRRKRGLFGA IAGFIEGGWQ 360
2.3.2.1a EPI_ISL_19836227 A/India/2025 321 YVKSNKLVLA TGLRNSPQKE ERRKKRGLFG AIAGFIEGGW 360
2.3.2.1c EPI_ISL_19850663 A/Vietnam/2025 321 YVKSSKLVLA TGLRNSPQRE RRRKKRGLFG AIAGFIEGGW 360
Highly pathogenic avian influenza viruses with H5 and H7 and hemagglutinin (HA) subtypes evolve from low-pathogenic precursors through acquisition of multiple basic amino acid residues (Arginine [R] or Lysine[K]) at the hemagglutinin (HA) cleavage site, the HA1/HA2 junction (29, 30). However, acquisition of a polybasic cleavage site alone is not sufficient to convert an influenza virus into a pandemic capable virus. The H5N1 viruses currently circulating in cattle and other animals have function polybasic cleavage sites at the HA1/HA2 junction (Table 1, polybasic cleavage sites are in blue). To date these viruses have not acquired the additional mutations required for sustained human-to-human transmission. For H5N1 influenza viruses to become human pandemic pathogens other changes, including reassortment, must occur in other genes besides the gene encoding for hemagglutinin, the surface glycoprotein (31). Multiple changes in Spike were important for the SARS-CoV-2 progenitors to become highly transmissible in humans. It is likely that changes in other genes were also required.
Acknowledgements
R.F.G. has been supported by NIH (R01AI132223, R01AI132244, U19AI142790, U54CA260581, U54HG007480, and OT2HL158260), the Coalition for Epidemic Preparedness Innovation, the Wellcome Trust Foundation, Gilead Sciences, and the European and Developing Countries Clinical Trials Partnership Programme. I thank Edward Holmes, Alex Crits-Christoph, Jonathan Pekar, Andrew Rambaut, Angie Rasmussen, Zach Hensel, and Stuart Neil for helpful comments.
References
- K. G. Andersen, A. Rambaut, W. I. Lipkin, E. C. Holmes, R. F. Garry, The proximal origin of SARS-CoV-2. Nat Med 26, 450-452 (2020).
- R. F. Garry, SARS-CoV-2 furin cleavage site was not engineered. Proc Natl Acad Sci U S A 119, e2211107119 (2022).
- H. Zhou et al., A Novel Bat Coronavirus Closely Related to SARS-CoV-2 Contains Natural Insertions at the S1/S2 Cleavage Site of the Spike Protein. Curr Biol 30, 2196-2203.e2193 (2020).
- B. A. Johnson et al., Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature 591, 293-299 (2021).
- B. A. Johnson et al., Furin Cleavage Site Is Key to SARS-CoV-2 Pathogenesis. bioRxiv : the preprint server for biology (2020).
- T. P. Peacock et al., The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nature microbiology 6, 899-909 (2021).
- M. A. Peña-Hernández et al., SARS-CoV-2-related bat viruses evade human intrinsic immunity but lack efficient transmission capacity. Nature microbiology 9, 2038-2050 (2024).
- J. E. Pekar et al., The recency and geographical origins of the bat viruses ancestral to SARS-CoV and SARS-CoV-2. Cell, (2025).
- S. Temmam et al., Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. Nature 604, 330-336 (2022).
- The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature microbiology 5, 536-544 (2020).
- S. Fujita et al., Virological characteristics of a SARS-CoV-2-related bat coronavirus, BANAL-20-236. EBioMedicine 104, 105181 (2024).
- S. Temmam et al., SARS‐CoV‐2‐related bat virus behavior in human‐relevant models sheds light on the origin of COVID‐19. EMBO reports 24, e56055 (2023).
- X. Ou et al., Host susceptibility and structural and immunological insight of S proteins of two SARS-CoV-2 closely related bat coronaviruses. Cell discovery 9, 78 (2023).
- J. Wang et al., SARS-related coronavirus S-protein structures reveal synergistic RBM interactions underpinning high-affinity human ACE2 binding. Sci Adv 11, eadr8772 (2025).
- F. R. Hills, J. L. Geoghegan, M. Bostina, Architects of infection: A structural overview of SARS-related coronavirus spike glycoproteins. Virology 604, 110383 (2025).
- F. Madeira et al., The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Research 52, W521-W525 (2024).
- W. Pearson. (1991 LALIGN/PLALIGN local alignments ).
- P. Simmonds, C→U transition biases in SARS-CoV-2: still rampant 4 years from the start of the COVID-19 pandemic. mBio 15, e02493-02424 (2024).
- Y. Kosugi et al., Molecular basis of sarbecovirus evolution and receptor tropism in natural hosts, potential intermediate hosts, and humans. bioRxiv : the preprint server for biology, 2025.2003.2022.644775 (2025).
- L. Kang et al., A selective sweep in the Spike gene has driven SARS-CoV-2 human adaptation. Cell 184, 4392-4400.e4394 (2021).
- S. Zhang et al., Loss of Spike N370 glycosylation as an important evolutionary event for the enhanced infectivity of SARS-CoV-2. Cell research 32, 315-318 (2022).
- C. Wang et al., Molecular mechanisms of RaTG13 and SARS-CoV-2 RBD bound to Rhinolophus affinis bat ACE2. Protein Science 34, e70117 (2025).
- C. Cereghino et al., Evolution at Spike protein position 519 in SARS-CoV-2 facilitated adaptation to humans. Npj Viruses 2, 29 (2024).
- X. L. Yang et al., Isolation and Characterization of a Novel Bat Coronavirus Closely Related to the Direct Progenitor of Severe Acute Respiratory Syndrome Coronavirus. J Virol 90, 3253-3256 (2015).
- D. J. Benton et al., The effect of the D614G substitution on the structure of the spike glycoprotein of SARS-CoV-2. Proceedings of the National Academy of Sciences 118, e2022586118 (2021).
- L. Yurkovetskiy et al., Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell 183, 739-751.e738 (2020).
- A. E. Stout, J. K. Millet, M. J. Stanhope, G. R. Whittaker, Furin cleavage sites in the spike proteins of bat and rodent coronaviruses: Implications for virus evolution and zoonotic transfer from rodent species. One health (Amsterdam, Netherlands) 13, 100282 (2021).
- A. G. Wrobel et al., SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nature structural & molecular biology 27, 763-767 (2020).
- T. Horimoto, Y. Kawaoka, Reverse genetics provides direct evidence for a correlation of hemagglutinin cleavability and virulence of an avian influenza A virus. J Virol 68, 3120-3128 (1994).
- M. Hatta, P. Gao, P. Halfmann, Y. Kawaoka, Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293, 1840-1842 (2001).
- O. Stech et al., Acquisition of a polybasic hemagglutinin cleavage site by a low-pathogenic avian influenza virus is not sufficient for immediate transformation into a highly pathogenic strain. J Virol 83, 5864-5868 (2009).