Since 2005, there have been eight public health emergency of international concern (PHEIC) declarations. The tow last are related to mpox outbreaks, the first one from 2022-2023with clade IIb that spread globally in more than 100 countries and more recently since 14th august 2024 an outbreak with clade Ib (WHO, 2024). The current declaration followed the declaration of a Public Health Emergency of Continental Security (PHECS) by Africa CDC on August 13th and both were consecutive to the concerning expansion of outbreaks with clade in DRC, especially clade Ib, firstly described in September 2023 in Kamituga, a mining city in the South Kivu province and driven by transmission through sexual contact (Masirika et al., 2024; Vakaniaki et al., 2024; WHO, 2023). Since 2023, the number of mpox cases in DRC peaked above 14000 and were reported from new foci in large urban areas and transportation hubs, including Kinshasa (the capital city of the DRC). The first cases described in 2023 in the capital were all clade Ia which were linked to strains circulating in Equateur, Sud-Ubangi and Maindombe’s provinces (Kinganda Lusamaki et al., 2024). Here, we present a first case of mpox infection with clade Ib in Kinshasa, DRC.
On July 1st, 2024, we investigated a young adult woman in Ngiri-Ngiri health zone who presented mpox like lesions. The patient reported no contact with living or death animals nor consumption of meat (smoked). However, she reported an occasional sexual contact with a long-time friend in Kinshasa prior the start of the lesions. The casual partner who lives in Matadi, 360 kilometers west of Kinshasa, reported a rash 3 days after the lesions appeared on the young adult woman while he was already in South Africa where he received treatment.
The patient (young adult women) showed a polymorphism of dermatological lesions, with at least 300 counted, dominated by pustular and papular lesions, apart from a few bullous lesions on the soles of the feet. Rashes started to appear around the genital area and upper limb, and gradually spread throughout the body. The lesions were predominantly on the face, upper limbs, trunk and a particularly aspect in genital area. On the face and lower limbs, the lesions were pustular, umbilicated and mostly surmounted by crusty lesions; on the other hand, the same lesions were more or less tense on the extremities of the upper limbs. In the anogenital region, there were a few isolated opalescent bullous lesions on the inner surfaces of the labia majora, associated with a swelling mimicking lymphedema of the labia majora, slightly tender to palpation; while on the pubis, bullous lesions overhung the labia majora, extending slightly towards the left labia majora. An erythematous scaly placard covered almost the entire perineo-inguinocrural region, marked by whitish coatings at the bottom of two inguinocrural folds.
In general, mucous membranes were affected, except the oral mucosa. Eyes were affected, mainly the left eye which showed mucopurulent secretions adherent to the eyelashes. Diffuse conjunctival hyperemia was more marked on the left and discrete on the right. Palpebral statics were conserved; loupe examination of the right eye showed diffuse conjunctival hyperemia inferiorly, and a clear cornea; whereas on the left eye, mucopurulent secretions were abundant in the conjunctival dead end and adherent to the eyelashes. In addition, there was a subconjunctival para-limbic nodule, located in the supero-nasal quadrant, and two others in the supero- and infero-nasal quadrants. The Fluorescein test was positive on peri-limbal mucocorneal lesions and submucosal nodules. Fundus examination was normal in both eyes, after observation by a specialist ophthalmologist.
Mucocutaneous swabs were collected from vaginal pustulobullous lesions and ophthalmic secretions. Viral DNA was extracted from these samples and tested positive for orthopox virus using Polymerase Chain reaction (PCR) (LightMix® Modular Monkeypox Virus, Roche, Basel, Switzerland).
For sequencing, multiplex PCR amplifications were performed using MPXV clade IIb-specific primers (PrimalScheme for reference genome MT903345) (Chen et al., 2023), which generate 2.5 kb amplicons covering the complete MPXV genome. The PCR products were then quantified using Qubit device with QubitTM 1X dsDNA High Sensitivity Assay kit (ThermoFisher Scientific). The sequencing libraries were prepared using covidseq kit on Illumina platform and loaded on the Miseq. CZid consensus pipeline allowed genome consensus generation. Squirrel pipeline was used for apobe3 analysis (GitHub - aineniamh/squirrel).
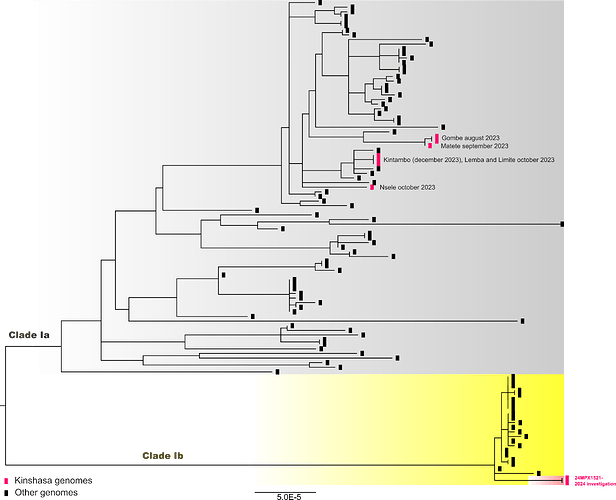
The sequences obtained from two samples from the same patient were closely related, homology, and clustered with MPXV clade Ib sequences from mpox cases detected in South Kivu province (Figure 1). Their position in the phylogenetic tree suggests they were part of the sustained human outbreak first reported in Kamituga health zone. The branch leading to our patient’s genomes has 10 SNPs including 6 APOBEC3-mediated mutations. However, the patient did not report travelling to the eastern part of the country or being in close contact or sexual contact with a partner coming from the eastern part of DRC, even though this cannot completely be excluded.
In conclusion, this is a confirmed case of MPXV clade Ib infection complicated by limbal nodular keratoconjunctivitis of the left eye and focal conjunctivitis of the right eye (viral superinfection); an abscess of the left labia majora of the vulva in a sexually active immunocompetent patient with a fragile integument of the genitocrural region due to persistent genitocrural candidiasis and long-term use of depigmenting topicals. Following previous alerts, the detection of clade Ib case in Kinshasa raises concerns on the current extent of the strain which was not expected to be circulating in the western DRC. Our exhaustive clinical description highlights the particularity of genital lesions, both in the chronology of the appearance of the first lesions and their atypical morphologies and risk of ocular complication. This clinical presentation increases the risk of sexual transmission. The absence of a link with eastern DRC where the mpox outbreak is predominated by a recent introduction and sustained human-to-human transmission of clade Ib suggests that this variant has already spread across the country. Its presence in Kinshasa, the capital city, with multiple international connections by air traffic and multiple exchanges with Brazzaville in the republic of Congo, illustrated the need for improved surveillance strategies to control the spread of the disease in other countries of west central African countries, like was the case in eastern DRC where clade Ib spread to neighboring countries in the east, like Burundi, Uganda, Kenya, and Ruanda and even reached Europe and Asia.
Figure 1 : A maximum likelihood tree constructed using IQ-Tree 2 with the HKY substitution model including 75 clade Ia (grey highlighted), 22 clade Ib (yellow highlighted) and 6 Kinshasa 2023 genomes. Three clade II genomes, KJ642615, KJ642616 and KJ642617 were used as outgroup and removed from the tree.
PARTNERS AND COLLABORATORS
The work is accomplished through the continuous, joined and coordinated efforts of partners supporting INRB and MPOX surveillance activities at the National level. Below are listed the institutions involved:
Institut National de Recherche Biomédicale (INRB), Kinshasa, Democratic Republic of the Congo: François Kasongo-Mulenda, Meris Matondo-Kuamfumu, Adrienne Amuri-Aziza, Eddy Kinganda-Lusamaki, Junior Bulabula-Penge, Anguy Makaka, Servet Kinbonza, Emmanuel Lokilo, Ola Rilia, Chloé Muswamba, Elisabeth Pukuta-Simbu, Gradi Luakanda, Prince Akil-Bandali, Princesse PAKU, Jean-Jacques Muyembe-Tamfum, Placide Mbala-Kingebeni, Antoine Nkuba-Ndaye, Steve Ahuka-Mundeke.
Hemorrhagic Fever and Monkeypox Program, Ministry of Health, Kinshasa, Democratic Republic of the Congo: Emile Malembi, Cris Kacita, Robert Shongo, Grâce Otema-Akenda, Cris Kacita-Osako.
TransVIHMI (Recherches Translationnelles sur le VIH et les Maladies Infectieuses), Université de Montpellier, Institut de Recherche pour le Développement (IRD), Institut National de Santé et de Recherche Médicale (INSERM), Montpellier, France : Ahidjo Ayouba, Eddy Kinganda-Lusamaki, Antoine Nkuba-Ndaye, Martine Peeters, Eric Delaporte.
Service de Microbiologie, Département de Biologie Médicale, Cliniques Universitaires de Kinshasa, Université de Kinshasa : Eddy Kinganda-Lusamaki, Meris Matondo-Kuamfumu, Anguy Makaka, Placide Mbala-Kingebeni, Jean-Jacques Muyembe-Tamfum, Antoine Nkuba-Ndaye, Steve Ahuka-Mundeke
Service de Dermatologie, Département de Spécialité, Cliniques Universitaires de Kinshasa, Université de Kinshasa : Sylvie Lundi-Kizela, Sabrina Kalonji-Tshilomba, Mohesa Iteke, Richard Nkwembe-Mpileng, Abrahaham Musuibwe, Véronique Kakiesse-Musumba.
Agence nationale de recherches sur le sida et les hépatites virales (ANRS) : Eric D’Ortenzio, Yazdan Yazdanpanah.
Service d’Ophtalmologie, Département de Spécialité, Cliniques Universitaires de Kinshasa, Université de Kinshasa : Deluxe Nsambayi-Lukusa.
STATEMENT ON CONTINUING WORK AND ANALYSES PRIOR TO PUBLICATION
The case description and its genome sequence are being shared pre-publication. Please note that this data is based on work in progress and should be considered preliminary. Our analyses of these data are ongoing and a publication communicating our findings on these. If you intend to use these data prior to our publication, please communicate with Dr François Kasongo, Dr Eddy Kinganda-Lusamaki, and Prof Steve Ahuka-Mundeke for coordination.
COMPETING INTERESTS
None of the other authors declare competing interests.
FUNDING
Agence Française de Dévelopement through the AFROSCREEN project (grant agreement CZZ3209, coordinated by ANRS-MIE Maladies infectieuses émergentes in partnership with Institut de Recherche pour le Développement (IRD) and Pasteur Institute) for laboratory support and PANAFPOX project funded by ANRS-MIE; E.L. received a PhD grant from the French Foreign Office. CZID for providing the reagents.
REFERENCES
Chen, N.F.G., Chaguza, C., Gagne, L., Doucette, M., Smole, S., Buzby, E., Hall, J., Ash, S., Harrington, R., Cofsky, S., Clancy, S., Kapsak, C.J., Sevinsky, J., Libuit, K., Park, D.J., Hemarajata, P., Garrigues, J.M., Green, N.M., Sierra-Patev, S., Carpenter-Azevedo, K., Huard, R.C., Pearson, C., Incekara, K., Nishimura, C., Huang, J.P., Gagnon, E., Reever, E., Razeq, J., Muyombwe, A., Borges, V., Ferreira, R., Sobral, D., Duarte, S., Santos, D., Vieira, L., Gomes, J.P., Aquino, C., Savino, I.M., Felton, K., Bajwa, M., Hayward, N., Miller, H., Naumann, A., Allman, R., Greer, N., Fall, A., Mostafa, H.H., McHugh, M.P., Maloney, D.M., Dewar, R., Kenicer, J., Parker, A., Mathers, K., Wild, J., Cotton, S., Templeton, K.E., Churchwell, G., Lee, P.A., Pedrosa, M., McGruder, B., Schmedes, S., Plumb, M.R., Wang, X., Barcellos, R.B., Godinho, F.M.S., Salvato, R.S., Ceniseros, A., Breban, M.I., Grubaugh, N.D., Gallagher, G.R., Vogels, C.B.F., 2023. Development of an amplicon-based sequencing approach in response to the global emergence of mpox. PLOS Biol. 21, e3002151. Development of an amplicon-based sequencing approach in response to the global emergence of mpox
Kinganda Lusamaki, E., Amuri Aziza, A., Fernandez Nunez, N., Makangara-Cigolo, J.-C., Pratt, C.B., Hasivirwe Vakaniaki, E., Hoff, N.A., Luakanda - Ndelemo, G., Akil-Bandali, P., Sabiti Nundu, S., Mulopo-Mukanya, N., Ngimba, M., Modadra-Madakpa, B., Diavita, R., Paku, P., Pukuta-Simbu, E., Merritt, S., O’Toole, A., Low, N., Nkuba-Ndaye, A., Kavunga-Membo, H., Shongo, R., Liesenborghs, L., Wawina-Bokalanga, T., Vercauteren, K., Mukadi-Bamuleka, D., Subissi, L., Muyembe, J.-J.T., Kindrachuk, J., Ayouba, A., Rambaut, A., Delaporte, E., Tessema, S.K., D’Ortenzio, E., Rimoin, A.W., Hensley, L.E., Mbala-Kingebeni, P., Peeters, M., Ahuka-Mundeke, S., 2024. Clade I Monkeypox virus genomic diversity in the Democratic Republic of the Congo, 2018 - 2024: Predominance of Zoonotic Transmission. medrix https://doi.org/10.1101/2024.08.13.24311951
Masirika, L.M., Udahemuka, J.C., Schuele, L., Ndishimye, P., Otani, S., Mbiribindi, J.B., Marekani, J.M., Mambo, L.M., Bubala, N.M., Boter, M., Nieuwenhuijse, D.F., Lang, T., Kalalizi, E.B., Musabyimana, J.P., Aarestrup, F.M., Koopmans, M., Oude Munnink, B.B., Siangoli, F.B., 2024. Ongoing mpox outbreak in Kamituga, South Kivu province, associated with monkeypox virus of a novel Clade I sub-lineage, Democratic Republic of the Congo, 2024. Eurosurveillance 29. https://doi.org/10.2807/1560-7917.ES.2024.29.11.2400106
Vakaniaki, E.H., Kacita, C., Kinganda-Lusamaki, E., O’Toole, Á., Wawina-Bokalanga, T., Mukadi-Bamuleka, D., Amuri-Aziza, A., Malyamungu-Bubala, N., Mweshi-Kumbana, F., Mutimbwa-Mambo, L., Belesi-Siangoli, F., Mujula, Y., Parker, E., Muswamba-Kayembe, P.-C., Nundu, S.S., Lushima, R.S., Makangara-Cigolo, J.-C., Mulopo-Mukanya, N., Pukuta-Simbu, E., Akil-Bandali, P., Kavunga, H., Abdramane, O., Brosius, I., Bangwen, E., Vercauteren, K., Sam-Agudu, N.A., Mills, E.J., Tshiani-Mbaya, O., Hoff, N.A., Rimoin, A.W., Hensley, L.E., Kindrachuk, J., Baxter, C., De Oliveira, T., Ayouba, A., Peeters, M., Delaporte, E., Ahuka-Mundeke, S., Mohr, E.L., Sullivan, N.J., Muyembe-Tamfum, J.-J., Nachega, J.B., Rambaut, A., Liesenborghs, L., Mbala-Kingebeni, P., 2024. Sustained Human Outbreak of a New MPXV Clade I Lineage in the Eastern Democratic Republic of the Congo. Nat. Med. Sustained human outbreak of a new MPXV clade I lineage in eastern Democratic Republic of the Congo | Nature Medicine
WHO, 2024. WHO Director-General declares mpox outbreak a public health emergency of international concern. [WWW Document]. URL WHO Director-General declares mpox outbreak a public health emergency of international concern (accessed 8.21.24).
WHO, 2023. Mpox (monkeypox)- Democratic Republic of the Congo [WWW Document]. URL Mpox (monkeypox) - Democratic Republic of the Congo.